Structural Research of Type IX Secretion Systems
The type IX secretion system (T9SS) is a complex translocator distributed in the Bacteroidetes phylum. It is responsible for secreting a variety of effector proteins across the outer membrane (OM) or providing a means of locomotion (gliding motility) for mobile bacteria. In periodontal pathogens such as Porphyromonas gingivalis, T9SS can translocate virulence factors with conserved C-terminal structural domains (CTDs) across the OM. In past research, the main components and structure of T9SS have been identified and its possible action mechanisms have been revealed.
Advances in research on the composition of the T9SS architecture
Research has identified at least 21 components of the T9SS that build core structures in the IM and OM, some play regulatory or auxiliary roles, and others are involved in post-translational modification of cargo proteins. The common T9SS structure includes 1. The transmembrane complex GldKLMN, consisting of two inner membrane (IM) proteins, GldL (or PorL) and GldM (or PorM), an OM-associated loop complex composed of GldK OM lipoproteins, and GldN periplasmic proteins. 2. The SprA (or Sov) OM translocator. 3. An attachment complex composed of PorU, PorV, and PorZ proteins. These proteins are assembled through a dense network of interactions.
Analysis of the mechanism of substrate (CTD) recognition based on the T9SS structure
In recent years, researchers have combined solution nuclear magnetic resonance (NMR) spectroscopy, modeling, and molecular dynamics (MD) simulations to propose a structural model for the PorV: RgpB-CTD complex. The structural analysis reveals the recognition of CTD by T9SS and the mechanism of RgpB-CTD interaction with PorV. The crystal structure shows that two flexible surface loops (L1, L3) in PorV extend into the SprA pore, the RgpB-CTD consists of seven β-strands folded into an Ig-like fold, and the CTD is anchored by binding to the PorV internal plug helix (iH) and the flexible N-terminus (nL). The C-terminal β-strand of each RgpB-CTD is flipped and exchanged with neighboring strands, an essential dynamic in CTD recognition.
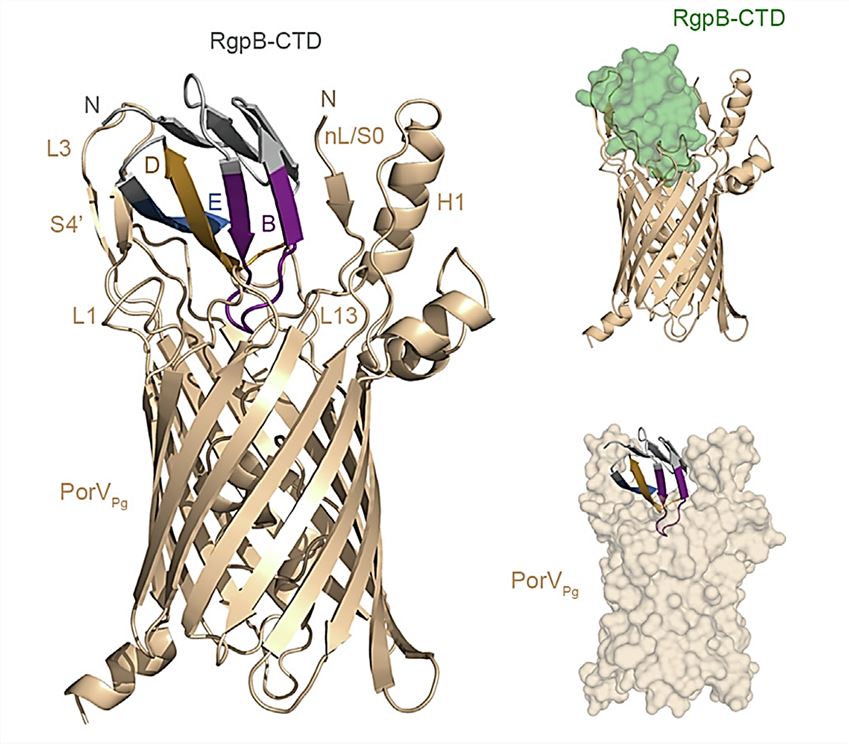 Figure 1. The model of the PorV: RgpB-CTD complex. (Dorgan B, et al., 2022)
Figure 1. The model of the PorV: RgpB-CTD complex. (Dorgan B, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Type 9 protein translocon Plug-complex | Flavobacterium johnsoniae | Cryo-EM single particle analysis | 3.7 Å | 6H3J |
| PorE C-terminal domain, a protein of T9SS | Porphyromonas gingivalis JCVI SC001 | X-ray diffraction | 1.55 Å | 6TOP |
| C-terminal part (residues 224-515) of PorM | Porphyromonas gingivalis | X-ray diffraction | 2.85 Å | 6EY5 |
| The N-terminal domain of PorA | Porphyromonas gingivalis ATCC 33277 | X-ray diffraction | 1.3 Å | 6KJK |
| Dimeric latent PorU | Porphyromonas gingivalis ATCC 33277 | X-ray diffraction | 3.35 Å | 6ZA2 |
| PorZ | Porphyromonas gingivalis | X-ray diffraction | 2.9 Å | 5M11 |
| The folded domain of PorN | Porphyromonas gingivalis ATCC 33277 | X-ray diffraction | 2 Å | 7PVH |
Table 1. Structural research of the type IX secretion systems.
Creative Biostructure invites researchers and pharmaceutical companies to collaborate and take advantage of our superior structural analysis services. With years of industry experience, we have pioneered innovative methods and cutting-edge technologies for the structural analysis of membrane proteins. Our team of scientists utilizes advanced techniques such as X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy to obtain high-resolution structural data, providing valuable insights into the structural and functional mechanisms of type IX secretion systems.
Whether your research focuses on membrane proteins or other biomolecules, we are the right partner for you. Contact us to learn how our capabilities can enhance your research and propel you toward your scientific goals.
References
- Dorgan B, et al. Structural Model of a Porphyromonas gingivalis type IX Secretion System Shuttle Complex. J Mol Biol. 2022. 434(23): 167871.
- Vincent MS, et al. Dynamic proton-dependent motors power type IX secretion and gliding motility in Flavobacterium. PLoS Biol. 2022. 20(3): e3001443.
