Structural Research of Drug/Metabolite Transporter (DMT) Superfamily
The drug/metabolite transporter (DMT) superfamily is a group of membrane proteins involved in the transport of a wide range of substances, including drugs and metabolites. These proteins are essential for various biological processes, such as nutrient uptake and cell signaling. Structural research of the DMT superfamily is crucial for understanding the mechanisms of their function and developing drugs that target these proteins.
The resistance to both chloroquine (CQ) and piperaquine (PPQ) has been linked to different sets of mutations in the P. falciparum chloroquine resistance transporter PfCRT, a member of the DMT superfamily. The structure of the PfCRT isoform from CQ-resistant, PPQ-sensitive South American 7G8 parasites was determined using cryo-electron microscopy and fragment antigen-binding technology.
The mutations responsible for CQ and PPQ resistance are located in moderately-conserved sites on distinct helices that line a central negatively-charged cavity, which is believed to be the main interaction site for positively-charged CQ and PPQ. The 7G8 isoform has similar affinities for both drugs and transports CQ in a pH- and membrane potential-dependent manner, indicating an active efflux mechanism is driving CQ resistance. However, it does not transport PPQ. The emergence of the F145I and C350R mutations in PfCRT, which are associated with reduced PPQ susceptibility in Asia and South America, were found to enable PPQ transport in 7G8 variant proteins and confer resistance in gene-edited parasites. The structural, functional, and computational analyses reveal unique mechanistic features that contribute to CQ and PPQ resistance in PfCRT variants. These results provide a comprehensive understanding of the molecular mechanism underlying the critical mediator of antimalarial treatment failures.
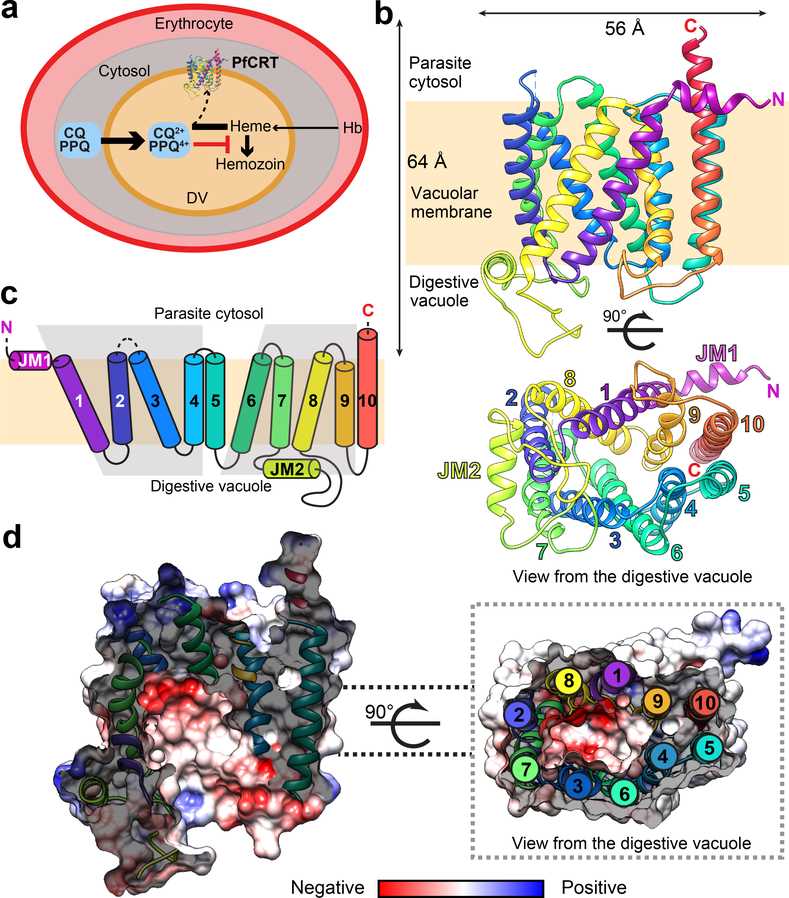 Figure 1. Cryo-EM structure of PfCRT 7G8. (Kim J, et al., 2019)
Figure 1. Cryo-EM structure of PfCRT 7G8. (Kim J, et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| YddG aromatic amino acid exporter (expressed in E. coli) | Starkeya novella | X-ray diffraction | 2.40 Å | 5I20 |
| Chloroquine Resistance Transporter 7G8 Isoform (expressed in HEK293 cells) | Plasmodium falciparum | Cryo-EM single particle analysis | 3.30 Å | 6UKJ |
Table 1. Structural Research of Drug/Metabolite Transporter (DMT) Superfamily.
Protein structural analysis is a crucial tool for understanding the function of membrane proteins like the DMT superfamily transporters. At Creative Biostructure, we provide a range of protein structural analysis services to help researchers study the structure and function of these proteins.
Our services include X-ray crystallography, cryo-electron microscopy (cryo-EM), and nuclear magnetic resonance (NMR) spectroscopy. X-ray crystallography is a powerful technique for determining the three-dimensional structure of proteins at high resolution. Cryo-EM is a rapidly developing technology that allows the determination of the structure of proteins at near-atomic resolution without the need for protein crystals. NMR spectroscopy is a powerful tool for studying the dynamics of proteins in solution.
If you are interested in exploring the structural research of the DMT superfamily and wish to learn more about the services we offer, please do not hesitate to contact us. Our team is always available to discuss your research needs and provide the best possible solutions for your project.
References
- Tsuchiya H, et al. Structural basis for amino acid export by DMT superfamily transporter YddG. Nature. 2016, 534(7607): 417-420.
- Kim J, et al. Structure and drug resistance of the Plasmodium falciparum transporter PfCRT. Nature. 2019, 576(7786): 315-320.