MemPro™ EPHA3
Creative Biostructure provides custom MemPro™ gene-to-structure services for EPHA3.
Erythropoietin-producing hepatocellular receptors (EPH receptors) are tyrosine kinases receptors that have essential functions including cell adhesion, migration, and axon guidance during development and homeostasis. Ephrins, as EPH receptors natural ligands, regulate stem cell differentiation and cell fate determination through interactions with EPH receptors. Depend on the sequence homologies and their binding affinity for their ligands, EPH family could be divided to EPHA (EPHA1-10) and EPHB (EPHB1-6).
Like the classical tyrosine kinase-receptor-mediated cell signaling, Eph--ephrin signaling functions wherein a cell bearing an Eph receptor upon binding to ephrinA ligand transmits signals downstream known as intracellular signaling. Eph--ephrin signaling plays vital role in the development of the nervous system and a wide range of functions such as the development of neuronal networks, axon guidance, formation and remodeling of synaptic connections, and nervous system repair. The Eph--ephrin interaction also regulates remodeling of vascular network formation during embryonic development.
EphA3 is expressed in embryonic tissues including the spinal cord, brain, axial muscles, kidneys, lungs, and heart and appears to play an essential role in epithelial-to-mesenchymal transition (EMT) Overexpression of EphA3 has been observed in some cancers, including leukemia, lymphoma, lung cancers, melanomas, and gastric carcinoma. Conversely, EphA3 mutations have been identified that suggest a tumor-suppressor role in some cancers.
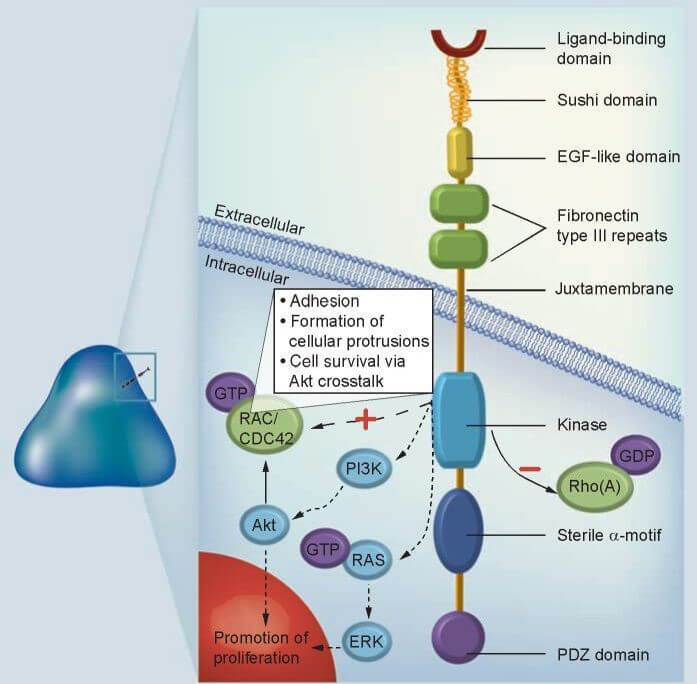
Figure 1. Structure of EPHA3 receptor
Glioblastoma (GBM) is the most common primary brain cancer. EphA3 knock down using small hairpin RNA proves that loss of epha3 prevents tumors formation and induces neuronal and glial cell differentiation. Additionally, EPHA3 is coexpressed with other highly proliferative markers like CD133, CD15 and integrin α6 on undifferentiated cells in tumor tissues. Meanwhile, another EPHA family member, EPHA2 is also frequently observed to coexpressed with EPHA3 and shows similar biological functions. Results confirmed that EPHA3, similar to EPHA2, limit MAPK signaling and thereby restricting differentiation and reduce proliferation.
However, Ephs are known for their context-dependent, dichotomous functions. It seems odd since overexpression in EphA3 is observed in various cancers. Typically, kinase-dormant Ephs promote cell–cell adhesion, invasion, and tumor (stem cell) maintenance and are regarded oncogenic, whereas Eph kinase activation is tumor suppressive by causing cell–cell segregation and reduced viability. Therefore, overexpression of kinase-dormant EphA3 in glioblastoma acts to maintain tumor cells in a dedifferentiated, tumorigenic state, whereas activation of its kinase inhibits glioma cell proliferation.
The EphA3-activating monoclonal antibody IIIA4 which targets bone marrow–derived mesenchymal/stromal and myeloid EphA3 positive cells in the tumor microenvironment, results in EphA3 phosphorylation, rapid cell contraction, and apoptosis. In xenograft models, IIIA4 treatment significantly inhibits tumor growth by disrupting the overall stromal and vascular tissue architecture and function.
References:
Day B W, Stringer B W, Al-Ejeh F, et al. EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme[J]. Cancer cell, 2013, 23(2): 238-248.
Vail M E, Murone C, Tan A, et al. Targeting EphA3 inhibits cancer growth by disrupting the tumor stromal microenvironment[J]. Cancer research, 2014, 74(16): 4470-4481.
