Coarse-grained Mempro™ Nanodisc Dynamics Simulation
Creative Biostructure has established a novel and advanced Mempro™ Nanodisc platform to study the membrane proteins, and the Mempro™ Nanodisc technology has made a great progress in the past decades. As more and more advanced techniques were emerged in the field of nanodisc studies, Creative Biostructure took advantage of the coarse-grained dynamics simulation to study the mechanism and structural details of nanodisc self-assembly in native-like lipid environment. Compared with the traditional experimental methods and all-atom dynamics simulation, Coarse-grained dynamics simulation has a lot of advantages in membrane protein research, and it can meet all the requirements of the customers to the maximum extent.
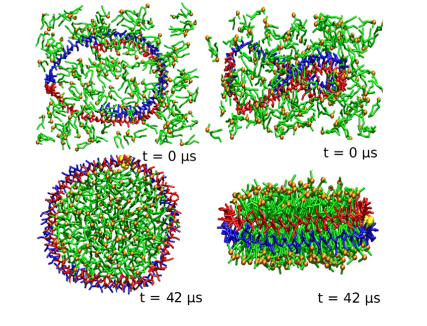 Figure 1. Schematic diagram of nanodisc self-assembly in CG-MD simulation. Initial configuration (top: left, top view; right, side view) and final configuration at 42 μs (bottom: left, top view; right, side view). Red and blue represent the two monomers 1 and 2 of aMSP1Δ, respectively. DMPC and phosphate headgroups are colored in green and orange, and lysine residues at position 90 are highlighted in yellow. Water molecules are not shown in the figure. (J. Phys. Chem. B 2015)
Figure 1. Schematic diagram of nanodisc self-assembly in CG-MD simulation. Initial configuration (top: left, top view; right, side view) and final configuration at 42 μs (bottom: left, top view; right, side view). Red and blue represent the two monomers 1 and 2 of aMSP1Δ, respectively. DMPC and phosphate headgroups are colored in green and orange, and lysine residues at position 90 are highlighted in yellow. Water molecules are not shown in the figure. (J. Phys. Chem. B 2015)
Coarse-grained Mempro™ Nanodisc Dynamics Simulation has the following advantages: (1) it can provide detailed insight into the structure of nanodisc self-assembly and the interactions of the MSPs with the lipids and with themselves, and the resolution can reach to the molecular level; (2) Compared with the all-atom Dynamics simulation, coarse-grained models make their time scales up to several microseconds, which ensures that the structure observed is in the equilibrium state; (3) The systems used in CG-MD can be much larger than the all-atom models.
Creative Biostructure has successfully applied coarse-grained dynamics simulation in a series of cases of nanodisc studies. The MARTINI force-field was used to define the parameters of the force field, and all simulations were performed with the GROMACS software. VMD was also indispensable for viewing all the dynamics process. We first described the self-assembly of membrane scaffold proteins into nanodiscs by using CG MD, and found that the configurational entropy of the lipids in the nanodisc was lower and the acyl tail order was higher than in a lamellar bilayer phase.
Coarse-grained Mempro™ Nanodisc Dynamics Simulation is usually combined with all-atom model to investigate the nanodisc systems, and this multiscale simulation approach has been widely used in various fields. We believe that computational studies will open a new window for future applications of nanodisc technology, including the characterization of both the properties of nanodisc phospholipid bilayers and nanodisc-embedded membrane proteins.
Coarse-grained Mempro™ Nanodisc Dynamics Simulation is quite an effective method for membrane protein research. Besides CG-MD, Mempro™ Nanodisc platform has also provides related services including AT-MD simulation of nanodiscs and nanodiscs model development. Please feel free to contact us for a detailed quote, We will make sure to offer you the best services and products.
References:
Debnath, et al. (2015). Structure and Dynamics of Phospholipid Nanodiscs from All-Atom and Coarse-Grained Simulations. Journal of Physical Chemistry B, 119(23): 6991-7002.
Monticelli, L., et al. (2008). The MARTINI Coarse-Grained Force Field: Extension to Proteins. Journal of Chemical Theory & Computation, 4(5): 819-834.
David, V.D.S., et al. (2005). GROMACS: fast, flexible, and free. Journal of Computational Chemistry, 26(16): 1701-1718.