Cell-free Membrane Protein Synthesis
Membrane proteins, a critical class of proteins responsible for functions such as signal transduction, molecular transport, and cellular communication, have been difficult to study due to their complex structures and cytotoxicity. Traditional research methods have encountered numerous challenges. However, the recent advances in Cell-Free Protein Synthesis (CFPS) technology have provided innovative solutions, allowing for the efficient and scalable production of membrane proteins in vitro. This article explores the advancements, methodologies, and future prospects of CFPS in the study of membrane proteins.
What is Cell-Free Protein Synthesis (CFPS)?
Cell-Free Protein Synthesis (CFPS) is a method that bypasses the need for living cells in protein production. Instead, it uses the cellular machinery extracted from a variety of sources, such as Escherichia coli, wheat germ, or mammalian cells, to facilitate transcription and translation in vitro. This method provides several advantages over traditional cell-based expression systems, including rapid protein production, the ability to express toxic proteins, and the flexibility to manipulate the expression environment.
- Rapid Production: CFPS can produce proteins within hours, compared to days or weeks required for cell-based systems.
- Expressing Toxic Proteins: Membrane proteins, which are often toxic to host cells, can be synthesized without affecting cell viability.
- Flexibility and Control: The open nature of CFPS allows for easy manipulation of reaction conditions, incorporation of non-natural amino acids, and the use of modified ribosomes and tRNAs.
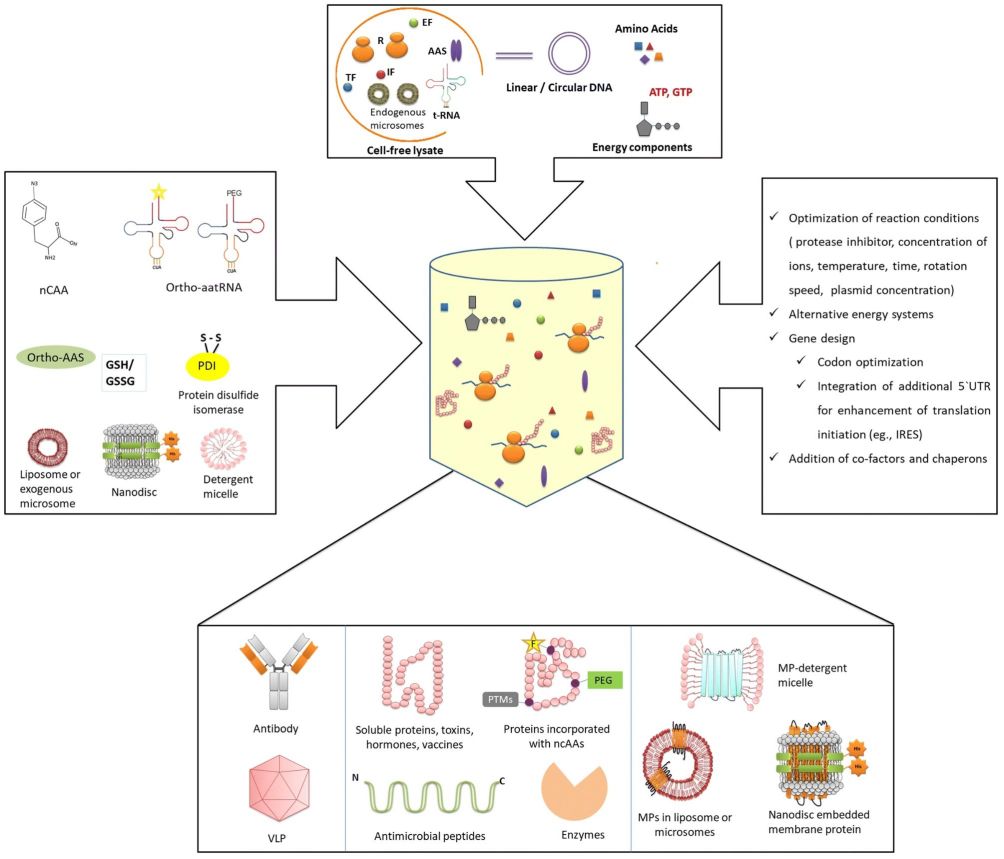 Figure 1. The overall process of cell-free protein synthesis. (Dondapati S K, et al., 2020)
Figure 1. The overall process of cell-free protein synthesis. (Dondapati S K, et al., 2020)
Synthesis of Membrane Proteins Using CFPS
Membrane Environment Augmentation
One of the groundbreaking aspects of CFPS in membrane protein research is the ability to supplement the reaction with membrane environments. This approach ensures that synthesized membrane proteins can fold properly and maintain their functional conformations. Two primary strategies have been developed to achieve this:
- Soluble Membrane Mimetics: These include detergents, liposomes, and nanodiscs that provide a suitable membrane-like environment for membrane protein synthesis and folding.
- Solid-supported Membrane Mimetics: These involve the use of lipid bilayers supported on solid surfaces, such as beads or chips, enabling the study of membrane proteins in a near-native state.
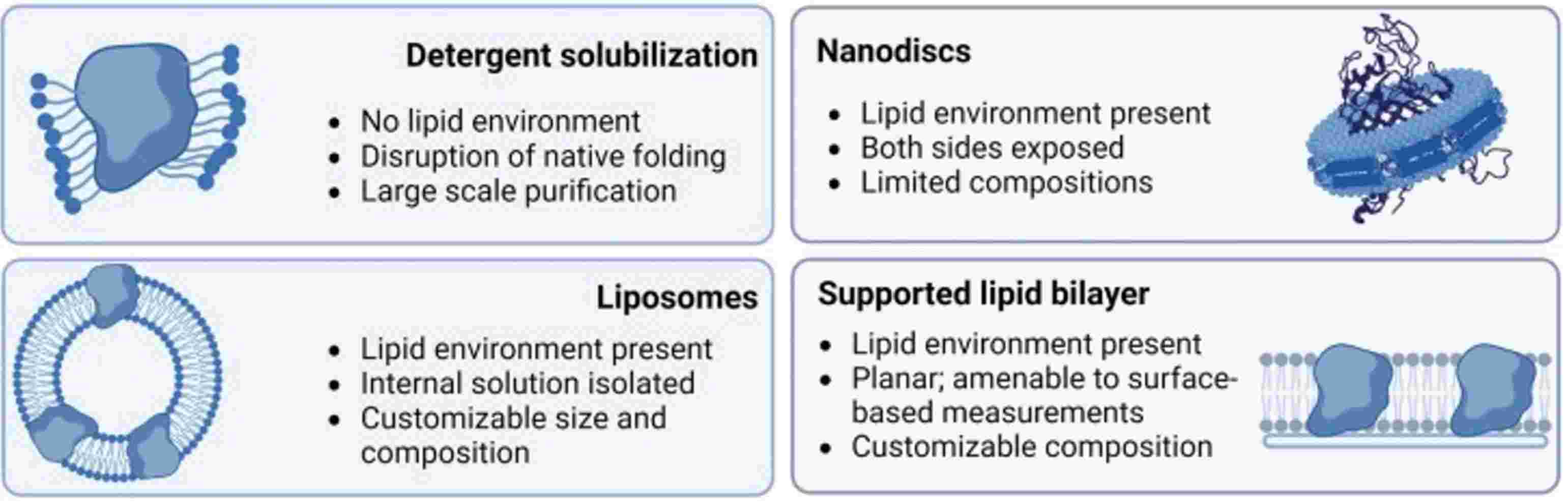 Figure 2. Comparison of different membrane environments for cell-free membrane protein synthesis. (Manzer Z A, et al., 2023)
Figure 2. Comparison of different membrane environments for cell-free membrane protein synthesis. (Manzer Z A, et al., 2023)
Techniques for Membrane Integration
Integrating membrane proteins into suitable environments is crucial for their proper folding and functionality. CFPS platforms have developed a variety of techniques to achieve this, providing flexibility and control over the membrane environment. The primary methods include the use of detergents, liposomes, nanodiscs, and supported lipid bilayers. Each of these techniques offers unique advantages and presents specific challenges:
| Techniques | Description | Advantages | Limitations |
| Detergent Solubilization | Detergents are amphiphilic molecules that can mimic the lipid bilayer environment by solubilizing membrane proteins. This technique is widely used due to its simplicity and ability to produce membrane proteins in relatively high yields. | Facilitates large-scale purification of membrane proteins. | Lacks a native lipid environment, leading to potential disruption of natural protein folding and functionality. |
| Liposome-Encapsulated CFPS | Liposomes are spherical vesicles composed of lipid bilayers, providing a more native-like environment for membrane proteins. They encapsulate synthesized proteins, mimicking natural cellular membranes. |
|
Stability and consistency can be challenging to maintain. |
| Nanodisc-Supported CFPS | Nanodiscs encapsulate a lipid bilayer within a membrane scaffold proteins (MSP), which simulates a membrane environment that is more controlled and stable compared to liposomes. |
|
Composition is limited and may not fully replicate the diversity of natural membranes. |
| Supported Lipid Bilayers | Supported lipid bilayers are planar structures that mimic cell membranes and are often used in surface-based analytical techniques. |
|
Planar configuration may not accurately represent the three-dimensional environment experienced by membrane proteins in vivo. |
Applications of CFPS in Membrane Protein Research
Structural Biology
CFPS has significantly impacted the field of membrane protein structural biology by providing a reliable method to produce large quantities of heterogeneous and difficult-to-express membrane proteins. This has enabled researchers to perform detailed structural analyses using techniques such as Cryo-Electron Microscopy (Cryo-EM) and Nuclear Magnetic Resonance (NMR) spectroscopy.
Case Study: GPCR Structure Determination
G protein-coupled receptors (GPCRs) are a major class of membrane proteins that play crucial roles in cellular signaling. Traditional methods of GPCR expression and purification have been challenging due to their low expression levels and instability. CFPS coupled with nanodiscs has facilitated the synthesis of functional GPCRs, allowing researchers to elucidate their structures and understand their mechanisms of action.
Functional Assays
Beyond structural studies, CFPS enables the development of functional assays to study membrane protein activity. By integrating membrane proteins into liposomes or nanodiscs, researchers can reconstitute and measure their functional properties, such as ligand binding, ion transport, and enzymatic activity.
Case Study: Ion Channel Functionality
Ion channels are essential membrane proteins that regulate the flow of ions across membranes, critical for cellular excitability and signaling. CFPS has been used to synthesize ion channels in a functional state, integrated into liposomes or nanodiscs. Functional assays have demonstrated the relevance of CFPS-produced ion channels, providing insights into their gating mechanisms and pharmacological profiles.
Innovations and Future Directions
- High-Throughput Screening (HTS): CFPS is ideal for HTS due to its scalability and speed, which accelerates drug discovery and functional genomics. Automation and microfluidics enable parallel synthesis and rapid analysis of thousands of membrane protein targets, significantly reducing research time and cost.
- Synthetic Biology and Engineering: CFPS is pivotal in synthetic biology, allowing the design and construction of novel membrane proteins with customized functions through the incorporation of non-natural amino acids and modular designs. This fosters new applications in biosensing, nanotechnology, and therapeutic development.
- Integration with Computational Methods: Combining CFPS with computational modeling and machine learning helps in the rational design of membrane proteins. Predictive models guide the optimization of CFPS protocols to achieve membrane proteins with desired properties, enhancing the efficiency of protein synthesis workflows.
Challenges and Solutions
- Protein Aggregation: Synthesized membrane proteins tend to aggregate, which can be mitigated by optimizing reaction conditions, using chaperones, and screening for stabilizing detergents and lipids.
- Scalability and Yield: Producing high yields of functional membrane proteins remains vital for practical applications. Improving extract preparation and reaction optimization are critical steps towards enhancing scalability and yield.
- Post-Translational Modifications (PTMs): Many membrane proteins require complex PTMs to function correctly. Achieving these modifications in CFPS is challenging but can be approached by using engineered extracts from eukaryotic cells or co-expressing necessary modifying enzymes.
Creative Biostructure provides custom membrane protein production and cell-free protein synthesis services to optimize and stabilize membrane proteins. Our expertise guarantees high-quality membrane proteins to support your research and drug development. Contact us for more information about our solutions.
References
- Kuruma Y, Ueda T. The PURE system for the cell-free synthesis of membrane proteins. Nature Protocols. 2015. 10(9): 1328-1344.
- Dondapati S K, Stech M, Zemella A, et al. Cell-free protein synthesis: a promising option for future drug development. BioDrugs. 2020. 34(3): 327-348.
- Manzer Z A, Selivanovitch E, Ostwalt A R, et al Membrane protein synthesis: no cells require. Trends in Biochemical Sciences. 2023. 48(7): 642-654.