MemPro™ HER3/ErbB3
Creative Biostructure provides custom MemPro™ gene-to-structure services for HER3/ErbB3.
The human epidermal growth factor receptor 3 (HER3/ErbB3) is a member of tyrosine kinases (RTK) family. HER family members play critical role in normal growth and development and their aberrantly activation are believed to correlated with oncogenic process. Along with other members such as HER2, EGFR and ErbB4, ErbB3 is frequently observed to overexpress in a wide variety of human cancers. Therefore, their transmembrane location and pro-oncogenic activity make them excellent targets for anti-cancer therapies.
Different with other HER family members, HER3/ErbB3 is the only member that has an impaired kinase domain, it has less than 1/1000th autophosphorylation activity compared with EGFR which suggests no ability to phosphorylate other proteins. Although amplified or overexpressed in some cancers, has not been reported to carry oncogenic mutations. Since ErbB3 homodimer should have no biological function, it often form heterodimers with other members, most notably ErbB2. When Heregulin (HRG, also called neuregulin (NRG)), erbB3 natural ligand, bind to the receptor, erbB3 will recruit other erbB2 receptor (usually erbB2) to form heterodimer. ErbB3 might be a major cause of treatment failure in cancer therapy, mainly through activation of the AKT and MAPK signaling pathway. Additionally, it also plays an important role in the development of various human cancers, including erbB2-overexpressing (erbB2+) breast cancer, castration-resistant prostate cancer (CRPC), platinum-resistant/refractory ovarian cancer, and EGFR tyrosine kinase inhibitor (TKI)-resistant non-small cell lung cancer.
Since the C-terminal tail of erbb3 contain the most tyrosine residues among all the HER members, once erbb3 is activated, the cascade of AKT and MAPK signaling would be strongly activated. They significantly promote cell proliferation and then leads to multidrug resistance in human cancers. Though erbb3 has proved its significant role in treatment resistance of cancer, lacking appreciate kinases activity makes small molecule tyrosine kinase inhibitors (TKIs) have quite limited effects. Additionally, mAb such as trastuzumab which specifically target erbb2 in breast cancer, also encountered the same problem.
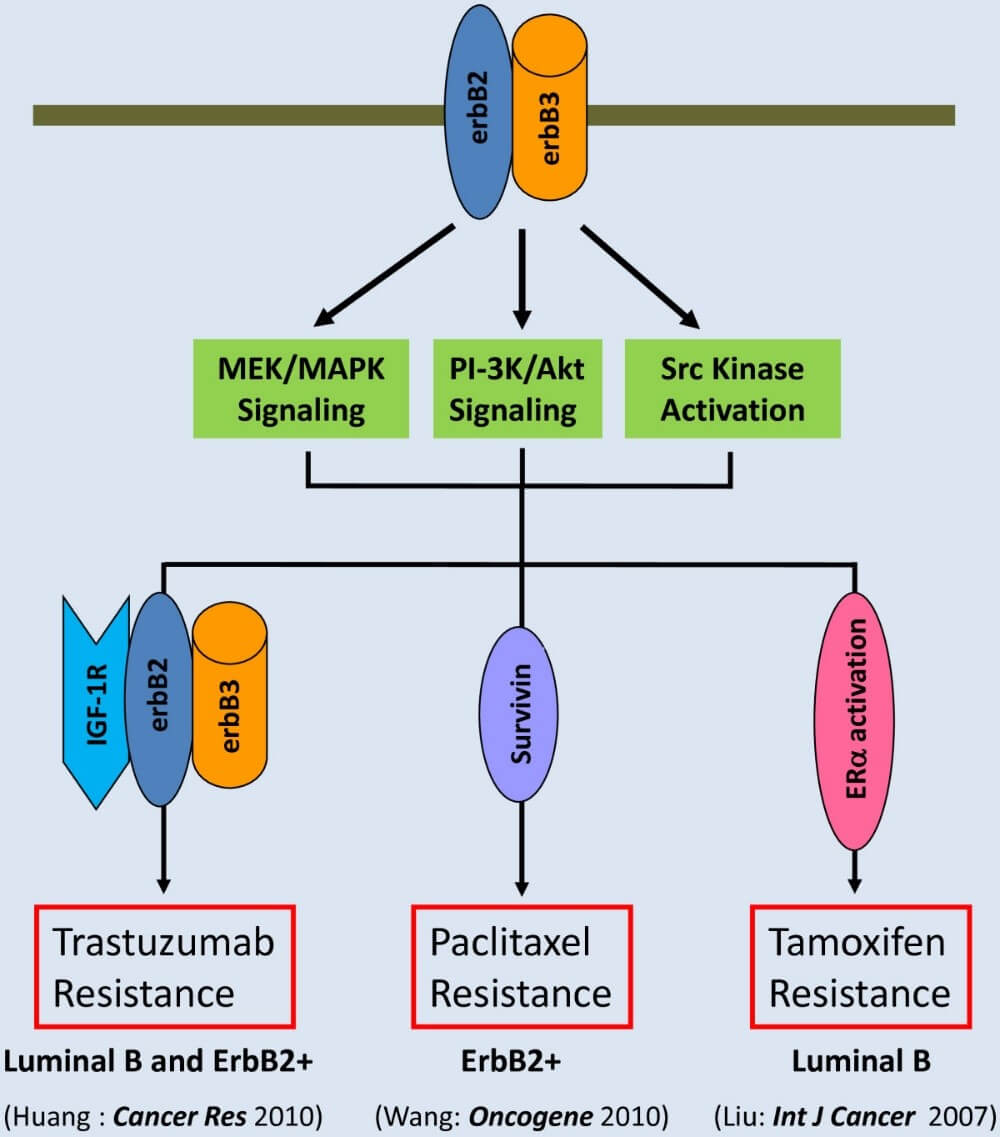 Figure 1. ErbB3 interacts with erbB2 to activate signaling pathways leading to multi-drug resistance in breast cancer (Ma et al. Molecular Cancer 2014, 13:105)
Figure 1. ErbB3 interacts with erbB2 to activate signaling pathways leading to multi-drug resistance in breast cancer (Ma et al. Molecular Cancer 2014, 13:105)
In order to eradicate cancerous cells, erbB3 must be effectively inhibited. Most erbB3-targeted therapies under development aim to inhibit erbB3 signaling by targeting the extracellular domain of the receptor. Several blocking antibodies are currently being investigated in preclinical. MM-121 and MM-111 (Merrimack Pharmaceuticals, Cambridge, MA) and U3-1287/AMG 888 (Amgen Inc., Thousand Oaks, CA) have shown significant antitumor activity in vitro and in vivo.
Bispecific monoclonal antibody is an artificial antibody which is composed of fragment from two distinct monoclonal antibodies and consequently binds to two different targets. Bispecific antibodies which have dual-target of erbB2/erbB3, or EGFR/erbB3 have shown promising potential in laboratory research and entered clinical trials. MM-111 is a bispecific antibody which target erB2/erbB3 to inhibit AKT pathway activation.
References:
Ma J, Lyu H, Huang J, et al. Targeting of erbB3 receptor to overcome resistance in cancer treatment[J]. Molecular cancer, 2014, 13(1): 1.
Jaiswal B S, Kljavin N M, Stawiski E W, et al. Oncogenic ERBB3 mutations in human cancers[J]. Cancer cell, 2013, 23(5): 603-617.