Structural Research of H+/Cl- or F- Exchange Transporters
H+/Cl- or F- exchange transporters, belonging to the CLC family of proteins, play a crucial role in cellular chloride and fluoride concentration regulation. These transporters encompass two distinct classes: Cl- channels and Cl-/H+ antiporters. Over the past few decades, extensive research efforts have been dedicated to unraveling the mysteries of their structural and functional characteristics.
A fundamental aspect of these H+/Cl- or F- exchange transporters lies in their conformational heterogeneity, especially concerning the critical glutamate residue, Gluex. Previous studies have proposed that the various conformations of Gluex may be responsible for the transport cycle in CLC-type Cl-/H+ antiporters. Structural snapshots of different CLC antiporters, such as CLC-ec1 from Escherichia coli and cmCLC from the thermophilic red alga Cyanidioschyzon merolae, have suggested the existence of three distinct Gluex conformations (Up, Middle, and Down).
To further delve into the heterogeneity of Gluex-conformations, a focused study was undertaken on CLC-ec1, one of the most extensively studied CLC antiporters. Utilizing crystal structures of the Gluex mutant E148D and wild-type CLC-ec1 with varying anion concentrations, the researchers successfully identified a novel "Midlow" conformation, representing a structural intermediate in the transport cycle.
The investigation into E148D mutant revealed intriguing results. Under conditions of high anion concentration, an additional anion was observed above the external Cl--binding site, suggesting the importance of anion concentration in modulating transporter dynamics. This finding highlights the intricate relationship between anion binding and transporter function.
Intriguingly, the study on the ungated E148A mutant brought to light the dynamic nature of the transporters. Here, a carboxylate in solution was found to occupy either the external or central Cl--binding site. This discovery, facilitated by the use of the anomalously detectable short carboxylic acid, bromoacetate, further strengthens the notion of conformational plasticity in CLC antiporters.
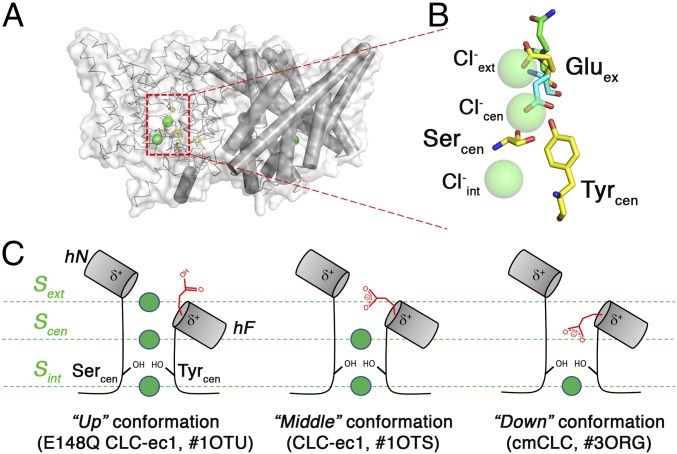 Figure 1. Multiple conformations of the Gluex residue in the transport cycle of CLC antiporters with known structures. (Park K,
et al., 2019)
Figure 1. Multiple conformations of the Gluex residue in the transport cycle of CLC antiporters with known structures. (Park K,
et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| ClC Chloride Channel (expressed in Escherichia coli) | Salmonella enterica subsp. enterica serovar Typhimurium | X-ray diffraction | 3.00 Å | 1KPL |
| ClC Chloride channel and Fab Complex (expressed in Escherichia coli) | Escherichia coli, Mus musculus | X-ray diffraction | 2.51 Å | 1OTS |
| EcClC-Fab complex in the absence of bound ions (expressed in Escherichia coli) | Escherichia coli, Mus musculus | X-ray diffraction | 3.20 Å | 2EXW |
| N- and C-terminal trimmed ClC-ec1 Cl-/H+ antiporter and Fab Complex (expressed in Escherichia coli) | Escherichia coli K-12, Mus musculus | X-ray diffraction | 2.40 Å | 4ENE |
| Engineered monomeric CLC-ec1 Cl-/H+ transporter (expressed in Escherichia coli) | Escherichia coli 042 | X-ray diffraction | 3.10 Å | 3NMO |
| EcCLC E148A mutant in Glutamate (expressed in Escherichia coli) | Escherichia coli K-12, Mus musculus | X-ray diffraction | 3.02 Å | 4FG6 |
| CLC-ec1 Fab Complex Cysless A399C-A432C mutant (expressed in Escherichia coli) | Escherichia coli K-12, Mus musculus | X-ray diffraction | 3.52 Å | 4MQX |
| E148D mutant CLC-ec1 in 20 mM bromide (expressed in Escherichia coli) | Escherichia coli K-12, Mus musculus | X-ray diffraction | 2.95 Å | 6AD7 |
| ClC-ec1 triple mutant (E113Q, E148Q, E203Q) (expressed in Escherichia coli) | Escherichia coli K-12 | X-ray diffraction | 2.62 Å | 6V2J |
| CLC transporter (expressed in Trichoplusia ni) | Cyanidioschyzon merolae | X-ray diffraction | 3.50 Å | 3ORG |
| A slow cyanobacterial Cl-/H+ antiporter (expressed in Escherichia coli) | Synechocystis sp. PCC 6803 | X-ray diffraction | 3.20 Å | 3ND0 |
| A CLC-type fluoride/proton antiporter (expressed in Escherichia coli) | Enterococcus casseliflavus EC10, synthetic construct | X-ray diffraction | 3.00 Å | 6D0J |
| CLC-7 (expressed in HEK293 cells) | Gallus gallus | Cryo-EM single particle analysis | 2.92 Å | 7JM6 |
| CLC-7/OSTM1 complex (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 2.82 Å | 7JM7 |
| CLC-7/Ostm1 membrane protein complex (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 3.70 Å | 7BXU |
| CLCa-type 2NO3-/1H+ antiporter (expressed in HEK293 cells) | Arabidopsis thaliana | Cryo-EM single particle analysis | 2.84 Å | 7XA9 |
Table 1. Structural research of H+/Cl- or F- exchange transporters.
At Creative Biostructure, we take pride in our state-of-the-art structural analysis services. With years of experience in the field, our team of expert researchers and advanced technologies allows us to perform comprehensive investigations into the structural dynamics of H+/Cl- or F- exchange transporters.
Our team comprises proficient scientists who leverage state-of-the-art technologies, such as cryo-electron microscopy (cryo-EM) and X-ray crystallography, to acquire high-resolution structural data. Contact us to explore the potential of our cutting-edge capabilities in empowering your research and bringing you closer to accomplishing your scientific objectives.
References
- Feng L, et al. Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science. 2010, 330(6004): 635-641.
- Park K, Lee B C, Lim H H. Mutation of external glutamate residue reveals a new intermediate transport state and anion binding site in a CLC Cl−/H+ antiporter. Proceedings of the National Academy of Sciences. 2019, 116(35): 17345-17354.