Structural Research of Transient Receptor Potential (TRP) Channels
Transient receptor potential (TRP) ion channels are composed of transmembrane proteins that act as sensors of cellular and environmental signals to detect and integrate a variety of physical and chemical stimuli. Research has found that TRP functions through changes in membrane potential induced by intracellular Ca2+ concentration and is associated with a variety of inherited and acquired human diseases. In recent years, the "resolution revolution" of cryo-electron microscopy has further elucidated the structure of TRP, laying the foundation for drug discovery and disease treatment related to TRP channels.
Classification of TRP
Most TRP families have antiquity, as well as a wide range of expression patterns and functions. The first trp gene was cloned from the Drosophila melanogaster retina, where it encoded a photo-transducing ion channel. Then, 27 members of the mammalian TRP channel superfamily have been identified, and mammalian TRPs have been classified into seven subfamilies based on sequence and topological differences, including TRPA, TRPC, TRPM, TRPN, TRPP, TRPV, and TRPML. It is a class of ion channels found in a wide range of tissues and cell types that are permeable to a wide range of cations, such as Ca 2+, Mg 2+, Na +, K +, etc.
Advances in research on TRP structure
Advances in the structural biology of TRP channels by cryo-electron microscopy have revealed a general as well as diverse set of structural components and regulatory sites for the TRP channel subfamily. The structure shows that TRP has a similar structure to voltage-gated ion channels, with six transmembrane structural domains (S1-S6), a pore-forming loop between S5 and S6, and intracellularly located C- and N-termini. the cytoplasmic end of the S6 helix opens and closes to regulate the entry of cations into the channel. the structural domains of S1-S4 control the pore, presumably in response to ligand binding, and the elements outside of the S5-S6 region act as linkers that control the gating element. Elements outside the S5-S6 region act as linkers that control gating elements.
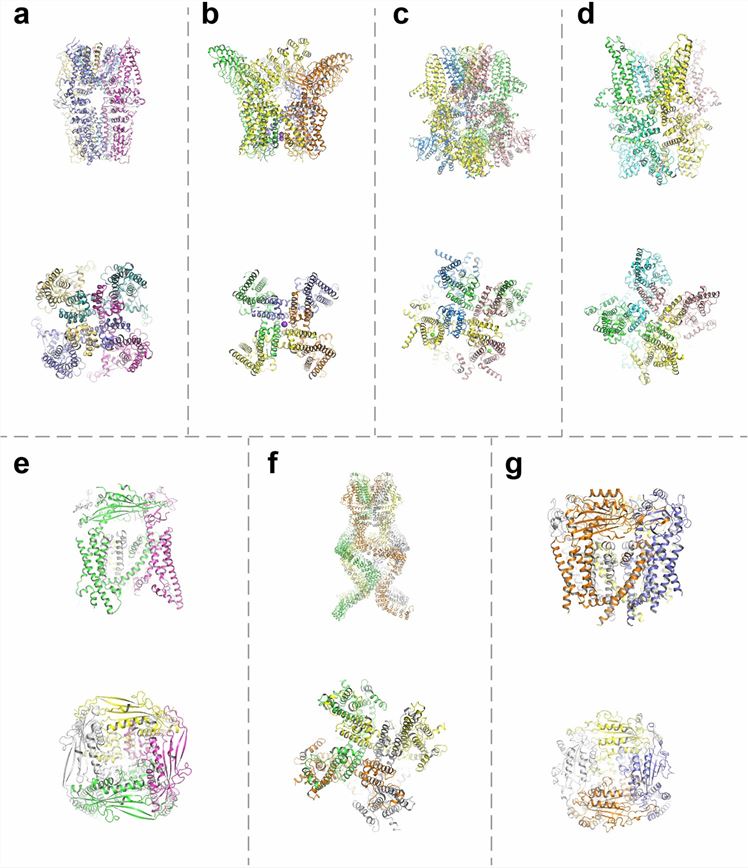 Figure 1. Structure of the TRP channel. a TRPA1; b TRPV1; c TRPM8; d TRPC3; e TRPML1; f TRPN; g TRPP2. (Zhang M, et al., 2023)
Figure 1. Structure of the TRP channel. a TRPA1; b TRPV1; c TRPM8; d TRPC3; e TRPML1; f TRPN; g TRPP2. (Zhang M, et al., 2023)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| TRPV3 | Mus musculus | Cryo-EM single particle analysis | 4.3 Å | 6DVW |
| TRPV3 channel in the apo conformation | Homo sapiens | Cryo-EM single particle analysis | 3.4 Å | 6MHO |
| TRPV3 channel in a putative sensitized conformation | Homo sapiens | Cryo-EM single particle analysis | 3.2 Å | 6MHS |
| TRPV3 in the presence of 2-APB in C4 symmetry | Homo sapiens | Cryo-EM single particle analysis | 3.5 Å | 6MHV |
| TRPV3 in complex with osthole | Mus musculus | Cryo-EM single particle analysis | 3.64 Å | 7RAS |
| TRPV3 in complex with the anesthetic dyclonine | Mus musculus | Cryo-EM single particle analysis | 3.16 Å | 7UGG |
| Thermo-sensitive TRP channel TRP1 in nanodiscs | Chlamydomonas reinhardtii | Cryo-EM single particle analysis | 3.45 Å | 6PW5 |
| Thermo-sensitive TRP channel TRP1 in detergent | Chlamydomonas reinhardtii | Cryo-EM single particle analysis | 3.53 Å | 6PW4 |
| Transient receptor potential (TRP) channel TRPV6 | Rattus norvegicus | X-ray diffraction | 3.247 Å | 5IWK |
| TRPV6-Y467A in amphipols | Homo sapiens | Cryo-EM single particle analysis | 4.34 Å | 6D7S |
| TRPV6-Y467A in complex with 2-Aminoethoxydiphenyl borate (2-APB) | Homo sapiens | Cryo-EM single particle analysis | 4.44 Å | 6D7T |
| TRPV6*-Y466A in complex with 2-Aminoethoxydiphenyl borate (2-APB) | Rattus norvegicus | X-ray diffraction | 3.497 Å | 6D7Q |
| TRPV6*- in complex with brominated 2-Aminoethoxydiphenyl borate (2-APB-Br) | Rattus norvegicus | X-ray diffraction | 4.3 Å | 6D7V |
| TRPV3-Y564A in open state at 37 degrees Celsius | Mus musculus | Cryo-EM single particle analysis | 4.48 Å | 6PVP |
| TRPV3-Y564A in intermediate state at 37 degrees Celsius | Mus musculus | Cryo-EM single particle analysis | 4.75 Å | 6PVQ |
| TRPV3 in MSP2N2 nanodiscs, closed state at 4 degrees Celsius | Mus musculus | Cryo-EM single particle analysis | 1.98 Å | 7MIJ |
| TRPV3 in MSP2N2 nanodiscs, sensitized state at 42 degrees Celsius | Mus musculus | Cryo-EM single particle analysis | 3.86 Å | 7MIL |
| TRPV3 in cNW11 nanodiscs, open state at 42 degrees Celsius | Mus musculus | Cryo-EM single particle analysis | 3.48 Å | 7MIO |
| TRPV6*-Y466A | Rattus norvegicus | X-ray diffraction | 3.37 Å | 6D7P |
| TRPV6*-Y466A- in complex with brominated 2-Aminoethoxydiphenyl borate (2-APB-Br) | Rattus norvegicus | X-ray diffraction | 3.6 Å | 6D7X |
| TRPV3 in complex with 2-Aminoethoxydiphenyl borate (2-APB) | Mus musculus | Cryo-EM single particle analysis | 4 Å | 6DVY |
| TRPV5 (1-660) in nanodisc | Oryctolagus cuniculus | Cryo-EM single particle analysis | 2.9 Å | 6O1N |
| Full-length TRPV5 in nanodisc | Oryctolagus cuniculus | Cryo-EM single particle analysis | 3 Å | 6O1P |
| TRPV5 with PI (4,5) P2 in nanodiscs | Oryctolagus cuniculus | Cryo-EM single particle analysis | 3.5 Å | 8FFO |
| TRPV5 W583A in nanodisc | Oryctolagus cuniculus | Cryo-EM single particle analysis | 2.8 Å | 6O1U |
| TRPV5 in complex with econazole | Oryctolagus cuniculus | Cryo-EM single particle analysis | 4.8 Å | 6B5V |
| ZINC17988990-bound TRPV5 in nanodiscs | Oryctolagus cuniculus | Cryo-EM single particle analysis | 3.78 Å | 6PBE |
| Cold- and menthol-sensing ion channel TRPM8 | Ficedula albicollis | Cryo-EM single particle analysis | 4.1 Å | 6BPQ |
| TRPV5 in nanodiscs in the presence of oleoyl coenzyme A, closed stated | Oryctolagus cuniculus | Cryo-EM single particle analysis | 3.09 Å | 8FHH |
| The canonical TRPC4 ion channel | Danio rerio | Cryo-EM single particle analysis | 3.6 Å | 6G1K |
Table 1. Structural research of the transient receptor potential (TRP) channels.
At Creative Biostructure, we offer various protein structural analysis services including X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy. These techniques allow us to determine the high-resolution structures of biomolecules, including transient receptor potential (TRP) channels, and provide insight into the action mechanisms. Our experienced scientists and technicians can provide customized structural analysis solutions, including protein expression, purification, crystallization, and data analysis.
We use cutting-edge equipment and software to ensure the highest quality results and fast turnaround times. Contact us today to learn more about our structural analysis services and how we can help you advance your research in protein structural biology. By working together, we can better understand the processes underlying cellular function and develop new therapies to treat various diseases.
References
- Zhang M, et al. TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases. Signal Transduct Target Ther. 2023. 8(1): 261.
- Cao E. Structural mechanisms of transient receptor potential ion channels. J Gen Physiol. 2020. 152(3): e201811998.
- Zhao Y, et al. Structural Pharmacology of TRP Channels. J Mol Biol. 2021. 433(17): 166914.
