Structural Research of O-Phosphatidyl Transferases
O-phosphatidyl transferases transfer non-canonical substitutions of phosphate groups to synthesize phospholipids as major components of membranes in cells and organelles. This process is essential for biological functions such as cellular metabolism, signaling, and immunity. In bacteria and lower eukaryotes, the precursor CDP-DAG is used in the de novo pathway for lipid synthesis through CDP-DAG alcohol O-phosphatidyl transferase. The crystal structure of phosphatidyl serine synthetase PSS reveals the catalytic mechanism of CDP-DAG-alcohol O-phosphoribosyl transferase.
Research progress on the structure of phosphatidylserine synthetase PSS
PSS catalyzes the formation of PS from CDP-DAG and serine. Crystallization of PSS in lipid cubic phase (LCP) and structural elucidation revealed that PSS is dimeric and completely embedded in the membrane. It has eight helices completely embedded in the membrane and one short horizontal helix (hH). Helixes 1-4 completely span the membrane, and the N-terminus of helix 5 and the C-terminus of helix 6 are located in the membrane core. The short β-strand between helices 4 and 5, and the β-strand between helices 6 and 7, form an antiparallel β-sheet on the cytoplasmic side.
Action mechanism of the CDP-DAG alcohol O-phosphatidyl transferase
Structural elucidation reveals that all substrates are present in the binding pocket and that residues of the ligand anion are highly conserved in CDP-AP. Chloride anions bound in the vicinity of the substrate play a special function in activating the substrate serine for nucleophilic attack. Site-directed mutagenesis studies of amino acid residues have shown that aspartate, alanine, and arginine are also all functionally related and play important roles in catalysis.
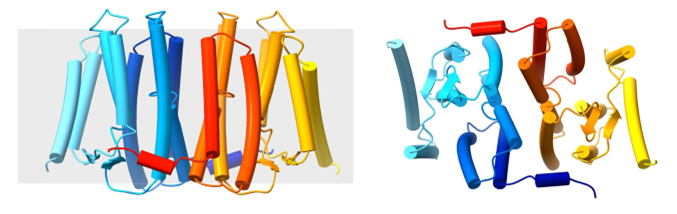 Figure 1. Side view and cytoplasmic view of PSS dimer. (Centola, M, et al., 2021)
Figure 1. Side view and cytoplasmic view of PSS dimer. (Centola, M, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Phosphatidyl serine synthase (PSS) in the closed conformation with bound citrate. | Methanocaldococcus jannaschii DSM 2661 | X-ray diffraction | 2.8 Å | 7B1N |
| Phosphatidyl serine synthase (PSS) in transition state. | Methanocaldococcus jannaschii DSM 2661 | X-ray diffraction | 2.51 Å | 7POW |
| Mannosylglycerate synthase: an archetypal mannosyltransferase | Rhodothermus marinus | X-ray diffraction | 1.95 Å | 2BO4 |
| Mannose-1-phosphate geranyl transferase from Thermus thermophilus HB8 | Thermus thermophilus HB8 | X-ray diffraction | 2.2 Å | 2CU2 |
| Phosphocholine Cytidylyltransferase from Streptococcus pneumoniae (LicC) | Streptococcus pneumoniae | X-ray diffraction | 1.5 Å | 1JYK |
| Mannosylglycerate synthase in complex with GDP-Mannose | Rhodothermus marinus DSM 4252 | X-ray diffraction | 2.7 Å | 2Y4M |
| Glucose-1-phosphate thymidylyltransferase, RmlA, complex with dTDP | Methanothermobacter thermautotrophicus | X-ray diffraction | 1.7 Å | 1LVW |
| Ribitol 5-phosphate cytidylyltransferase (TarI) | Bacillus subtilis subsp. spizizenii str. W23 | X-ray diffraction | 1.772 Å | 4JIS |
| CMP-sialic acid synthetase | Neisseria meningitidis | X-ray diffraction | 1.75 Å | 6CKJ |
| CMP-sialic acid synthetase in the presence of CTP and Ca2+ | Neisseria meningitidis | X-ray diffraction | 1.8 Å | 6CKK |
| Cytoplasm serine hydroxymethyltransferase (SHMT) | Komagataella phaffii CBS 7435 | X-ray diffraction | 2.5 Å | 5Z0Y |
Table 1. Structural research of O-phosphatidyl transferases.
Creative Biostructure has a long-standing commitment to structural biology and membrane protein research. We have advanced equipment and experienced experts in membrane protein structure determination and provide our clients with protein structural analysis services using NMR spectroscopy, X-ray crystallography and cryo-electron microscopy (cryo-EM). If you are interested in our services, please contact us for more details.
References
- Centola, M, et al. Crystal structures of phosphatidyl serine synthase PSS reveal the catalytic mechanism of CDP-DAG alcohol O-phosphatidyl transferases. Nat Commun. 2021. 6982.
- Nogly, P., et al. X-ray structure of a CDP-alcohol phosphatidyltransferase membrane enzyme and insights into its catalytic mechanism. Nature communications. 2014. 5, 4169.