Structural Research of Formate/Nitrite Transporter (FNT) Family
Secondary active transporters play a crucial role in the specific translocation of hydrophilic and/or charged compounds across biofilms. On the cell membrane, the formic acid nitrite transporter (FNT) forms channels or transport proteins. Formate is a metabolic product that participates in various cellular processes, including energy generation and carbon source metabolism. Nitrate is a nitrogen metabolite that participates in important nitrogen metabolism pathways in bacteria.
FNT family proteins maintain normal cell metabolism and function by transporting formate and nitrite from inside or outside the cell to the other side across the cell membrane. This is done to maintain the balance inside and outside the cell. FNT belongs to the major internal protein (MIP) superfamily, and its family members have been determined from gram-negative and gram-positive bacteria, archaea, yeast, plants, and lower Eukaryotes.
Three prokaryotic FNT subfamilies have been characterized: NirC, a channel necessary for nitrite absorption; FocA, a bidirectional formic acid channel; HSC (also known as FNT3 or AsrD) is a hydrogen sulfide channel necessary for substrate output. The available crystal structures of five representatives of these subfamilies reveal the homopentamer structure, which has a five-fold symmetry axis perpendicular to the membrane and has significant structural similarity to Aquaporin. Each monomer is a twisted bundle composed of six transmembrane spirals, through which a narrow permeable hole is connected to the cytoplasm and periplasm through two funnel-shaped inlets. The central chamber is defined by two contraction sites composed of hydrophobic residues, in which the highly conserved Histidine residue represents the only polar residue.
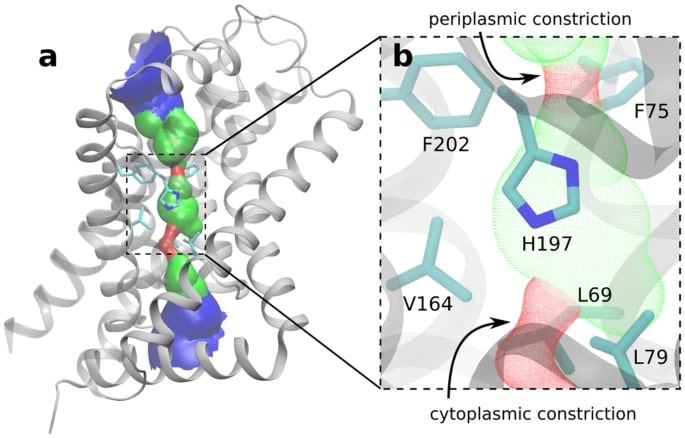 Figure 1. Crystal structure of a NirC monomer. (Kalina, et al., 2017)
Figure 1. Crystal structure of a NirC monomer. (Kalina, et al., 2017)
| Protein | Organism | Method | Resolution | PDB Entry ID |
|---|---|---|---|---|
| FocA, pentameric aquaporin-like formate transporter: Escherichia coli | Escherichia coli O157:H7 | X-ray diffraction | 2.24 Å | 3KCU |
| FocA formate transporter without formate: Vibrio cholerae | Vibrio cholerae | X-ray diffraction | 2.10 Å | 3KLY |
| FocA formate transporter at pH 4.0: Salmonella typhimurium | Salmonella enterica subsp. enterica serovar Typhi str. CT18 | X-ray diffraction | 2.80 Å | 3Q7K |
| FNT3 Hydrosulphide Channel (HSC), pH 9.0: Clostridium difficile | Clostridioides difficile 630 | X-ray diffraction | 2.20 Å | 3TDO |
| Formate-nitrite transporter PfFNT: Plasmodium falciparum | Plasmodium falciparum 3D7 | X-ray diffraction | 2.56 Å | 6VQQ |
Table 1. Structural research of formate/nitrite transporter (FNT) family.
Structural research is crucial to explore the function and regulatory mechanism of FNT family proteins, as well as their importance in biological systems. By analyzing the structure of FNT family proteins, key features such as channel formation, ion selectivity, substrate binding sites, and functional regions can be revealed, thereby promoting a deeper understanding of their functions and biological significance. This helps to provide new targets and theoretical foundations for drug development and treatment strategy design.
Creative Biostructure is a leading biotechnology company focusing on structural biology. We provide various services related to the research of FNT family protein structures, such as X-ray crystallography, cryo-electron microscopy (Cryo-EM), and nuclear magnetic resonance (NMR) services. By providing high-quality protein structure analysis services, our professional team can help you deeply understand the structure and function of FNT family proteins and provide support and cooperation opportunities for research and application in related fields. If you are interested in studying the structure of FNT proteins, you can contact us at any time and let us work together to explore the structure and function of FNT and make contributions to scientific progress.
Reference
- Kalina, et al. Energetics and mechanism of anion permeation across formate-nitrite transporters. Scientific Reports. 2017, 7(1).