Proteins Tags
In the vast landscape of molecular biology, one of the most important developments in the field of protein expression and purification is the use of protein tags. These tags, short peptide sequences or fusion partners, are attached to recombinant proteins to facilitate their isolation, detection, or functional analysis. By conferring specific properties to the proteins to which they are attached, protein tags have revolutionized research in molecular biology, enzymology, and biotechnology. Whether the goal is to purify a recombinant protein, enhance its solubility, or study its interactions, researchers have long relied on these tags to simplify and improve experimental workflows. Some widely used types include His-tag, MBP-tag, Flag-tag, SUMO-tag, and c-Myc-tag.
At Creative Biostructure, we offer protein purification services for various tagged proteins tailored to your project needs.
Overview of Protein Tags
Protein tags are small peptide sequences or proteins that are genetically fused to the target protein, offering a series of valuable advantages for researchers. Typically, these tags are short, ranging from a few amino acids to several dozen residues in length, ensuring that they do not interfere with the overall structure or function of the recombinant protein. Their inclusion can serve multiple purposes, including protein purification, detection, solubility enhancement, and facilitating interactions with other molecules.
The expression of recombinant proteins has long been a challenge, especially when dealing with low yields, difficult-to-purify proteins, or those that tend to aggregate. Protein tags are often used to circumvent these issues, providing both a method of isolation and a means of enhancing the functional properties of proteins in a cell-free or cellular system.
Beyond protein purification, protein tags are essential tools for biochemical analysis. They can be used to study protein-protein interactions, subcellular localization, post-translational modifications, or even to understand the molecular basis of disease. The versatility of protein tags makes them indispensable in a wide range of applications, from basic research to industrial biotechnology.
 Figure 1. Applications of tagged proteins.
Figure 1. Applications of tagged proteins.
Common Protein Tags
GST-tagged Proteins
The Glutathione S-Transferase (GST) tag is a fusion protein tag derived from the glutathione S-transferase enzyme. With a size of approximately 26 kDa, the GST tag is relatively large and is commonly used for affinity purification and improving protein solubility. This tag binds specifically to glutathione immobilized on a matrix, allowing efficient purification of tagged proteins. The GST tag is also beneficial for enhancing the expression and solubility of recombinant proteins, making it a versatile tool in molecular biology. Additionally, it can be cleaved off post-purification to yield the native target protein.
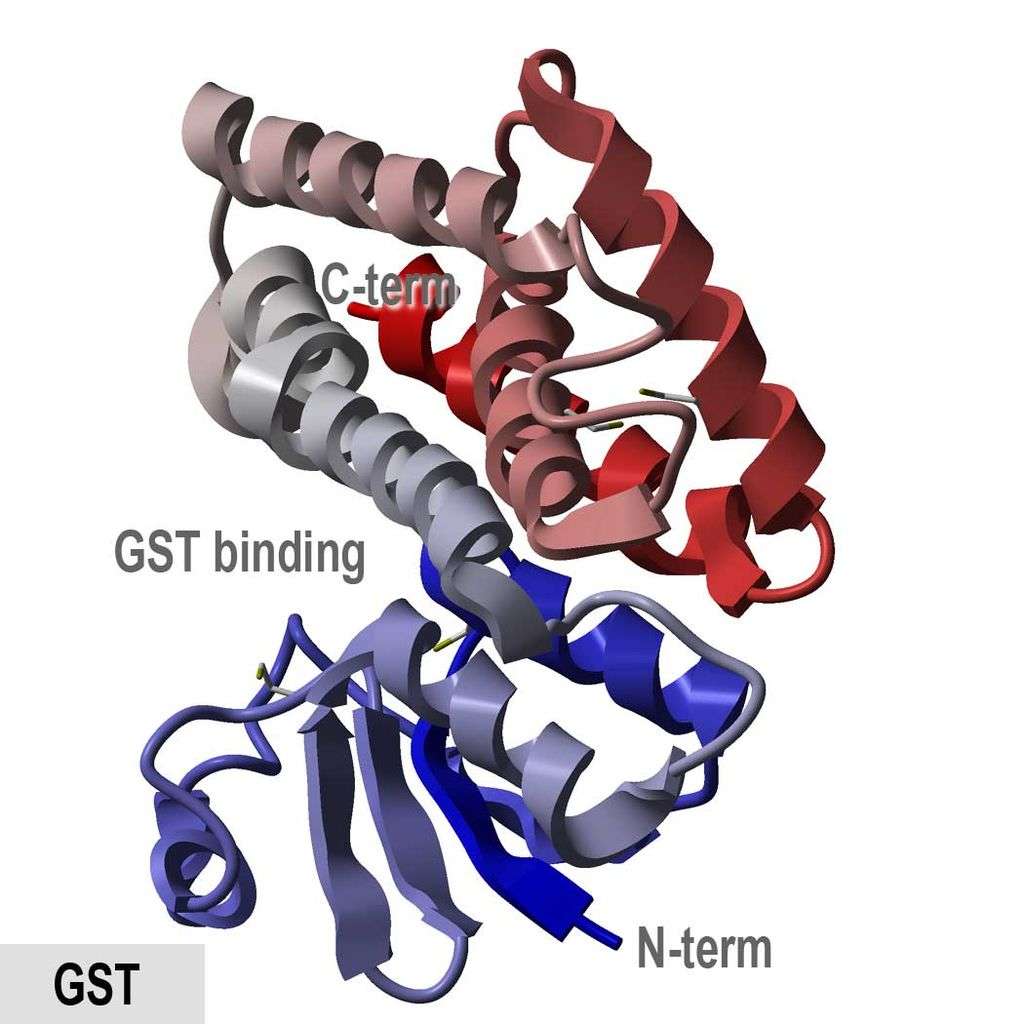 Figure 2. Crystallographic structure of glutathione S-transferase from Anopheles cracens. PDB code: 1R5A.
Figure 2. Crystallographic structure of glutathione S-transferase from Anopheles cracens. PDB code: 1R5A.
His-tagged Proteins
Perhaps the most ubiquitous and widely used protein tag is the His-tag, a sequence of six to ten histidine residues (His6-tag) attached to the N- or C-terminus of a protein. This small but highly charged tag has a high affinity for metal ions, particularly nickel, cobalt and copper, making it ideal for metal affinity chromatography (nickel-nitrilotriacetic acid (Ni-NTA) columns).
The His-tag is ideal for high-throughput screening, rapid protein purification, and applications where only moderate protein yields are required. It is a go-to choice for many researchers in academia and industry alike.
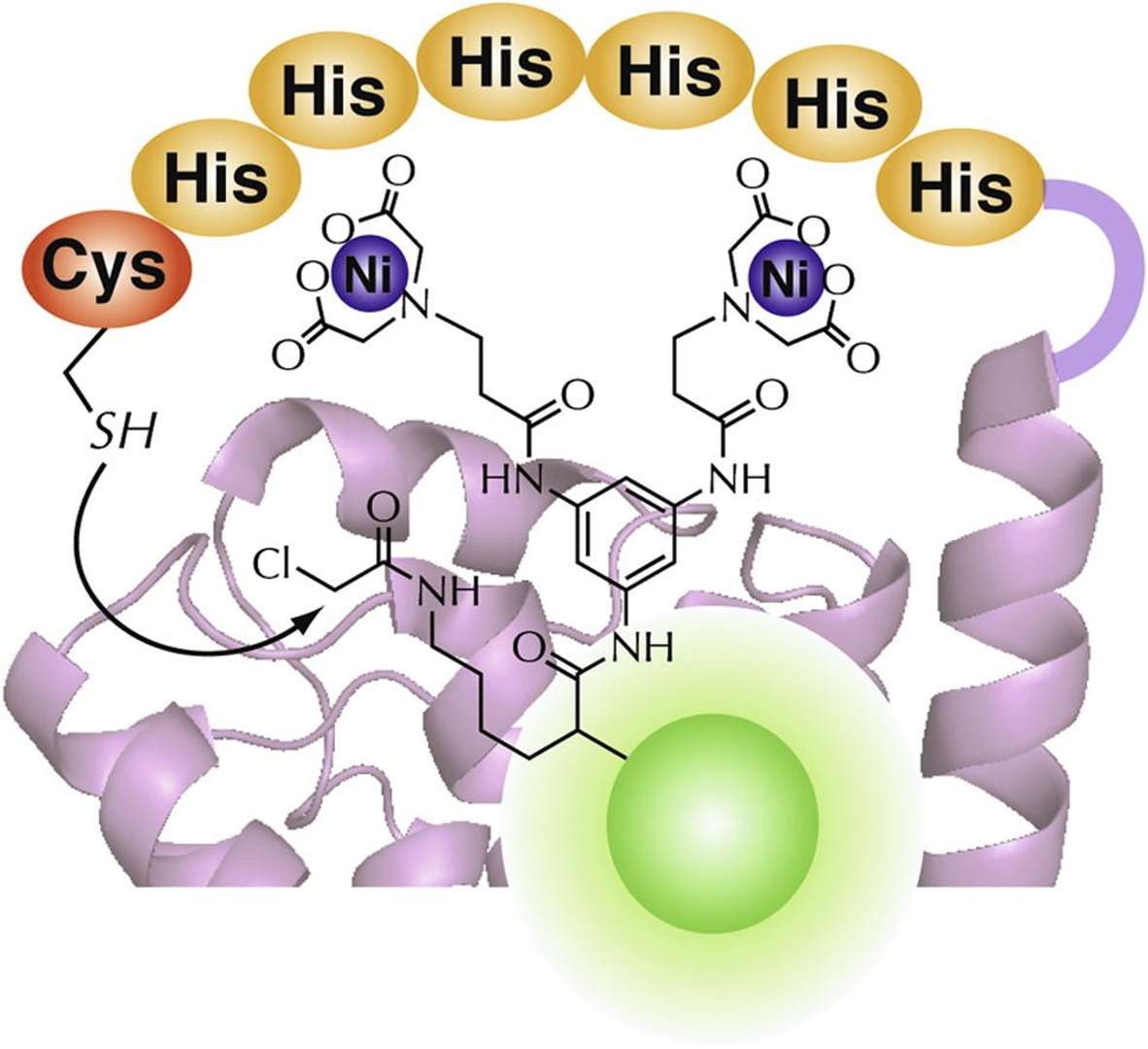 Figure 3. His-tag fused proteins with a binuclear Ni(II)–iminodiacetic acid (IDA) complex for selective recognition and covalent labeling. (Takahira et al., 2014)
Figure 3. His-tag fused proteins with a binuclear Ni(II)–iminodiacetic acid (IDA) complex for selective recognition and covalent labeling. (Takahira et al., 2014)
MBP-tagged Proteins
The MBP (Maltose Binding Protein) tag is another commonly used fusion protein, especially when solubility enhancement is a primary concern. MBP is a large protein (approximately 42 kDa) derived from Escherichia coli that binds maltose with high affinity. This tag is used for its ability to increase the solubility of the target protein, a benefit that is particularly valuable when expressing difficult-to-solubilize or aggregation-prone proteins.
MBP-tagged proteins are commonly used to enhance solubility in recombinant protein expression systems, especially for proteins that are difficult to express in soluble forms.
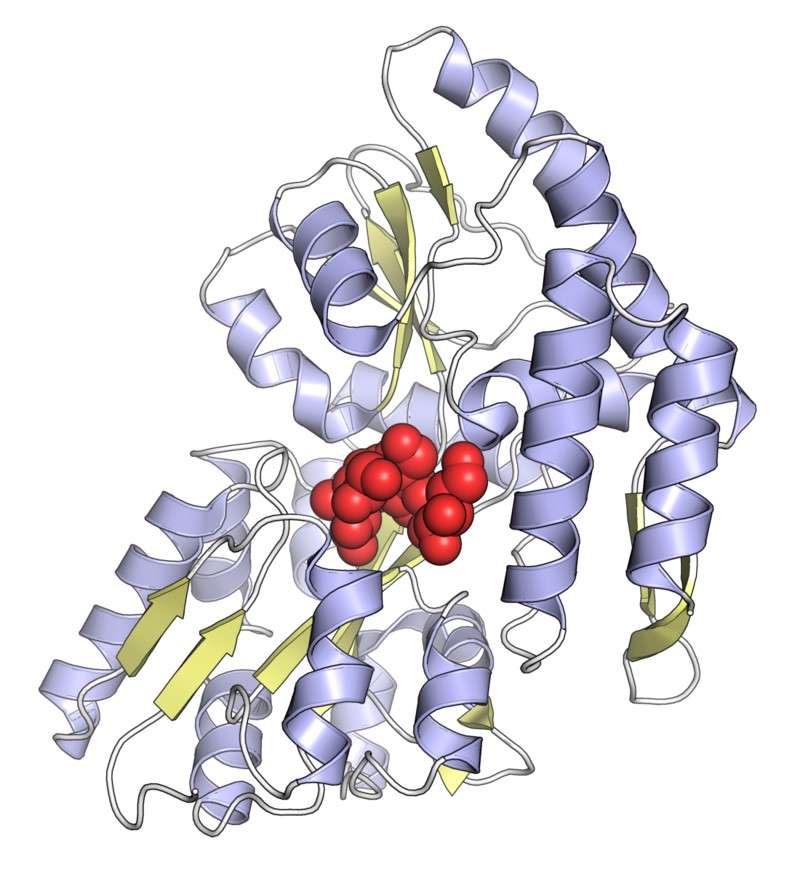 Figure 4. Maltose-binding protein from Escherichia coli, with a bound sugar molecule shown as red spheres. PDB code: 1FQC.
Figure 4. Maltose-binding protein from Escherichia coli, with a bound sugar molecule shown as red spheres. PDB code: 1FQC.
Flag-tagged Proteins
The Flag-tag is an 8-amino acid peptide tag derived from the human influenza virus hemagglutinin (HA) protein sequence. This short and highly specific sequence (DYKDDDDK) can be used for purification, detection, and interaction studies and is particularly valued for its minimal effect on protein function. Flag-tags are very useful in protein detection, interaction studies and purification where minimal perturbation of protein structure is essential.
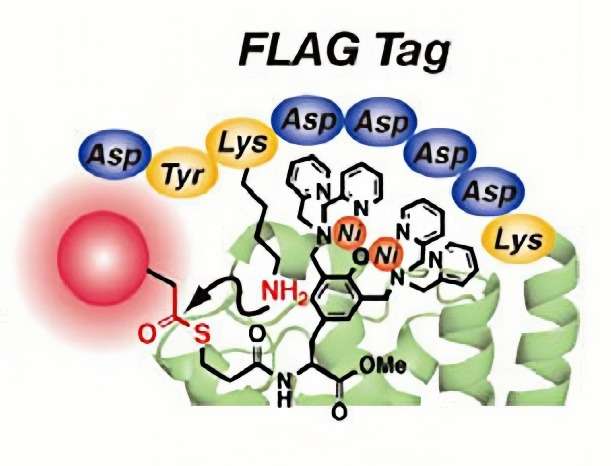 Figure 5. FLAG-tag selective covalent protein labeling via a binding-induced acyl-transfer reaction. (Nonaka et al., 2009)
Figure 5. FLAG-tag selective covalent protein labeling via a binding-induced acyl-transfer reaction. (Nonaka et al., 2009)
SUMO-tagged Proteins
The SUMO-tag (Small Ubiquitin-like Modifier) is a unique protein tag based on a small (around 12 kDa) ubiquitin-like protein. This tag is particularly valuable for improving protein solubility, stability, and in some cases, enhancing the biological activity of the target protein. SUMO-tagged proteins are often used in the context of protein-protein interaction studies, as the SUMO system mimics the behavior of ubiquitin, facilitating interaction with specific cellular machinery. SUMO tags are best suited for applications requiring protein stability, interaction studies, and in vivo-like expression systems.
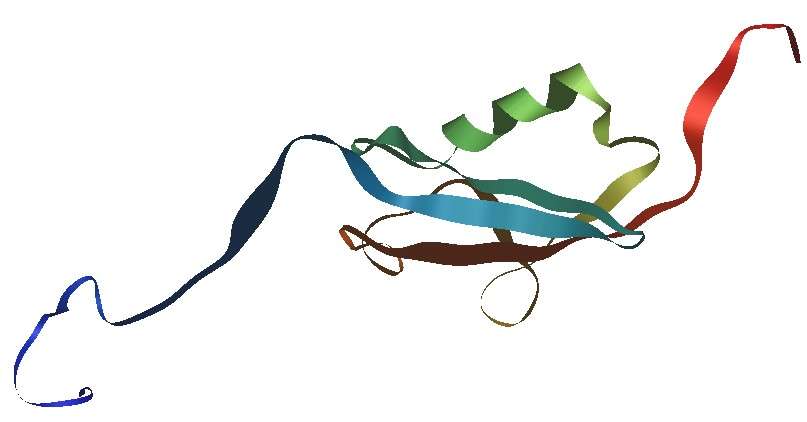 Figure 6. Structure schematic of human SUMO1 protein made with iMol and based on PDB file 1A5R, an NMR structure; the backbone of the protein is represented as a ribbon, highlighting secondary structure; N-terminus in blue, C-terminus in red.
Figure 6. Structure schematic of human SUMO1 protein made with iMol and based on PDB file 1A5R, an NMR structure; the backbone of the protein is represented as a ribbon, highlighting secondary structure; N-terminus in blue, C-terminus in red.
c-Myc-tagged Proteins
The c-Myc-tag is derived from a portion of the c-Myc protein, a well-known oncogene. This tag (EQKLISEEDL) is commonly used to detect and purify proteins, particularly in studies related to cell signaling and cancer research. c-Myc-tagging is widely used in cell biology, protein interaction, and cancer research.
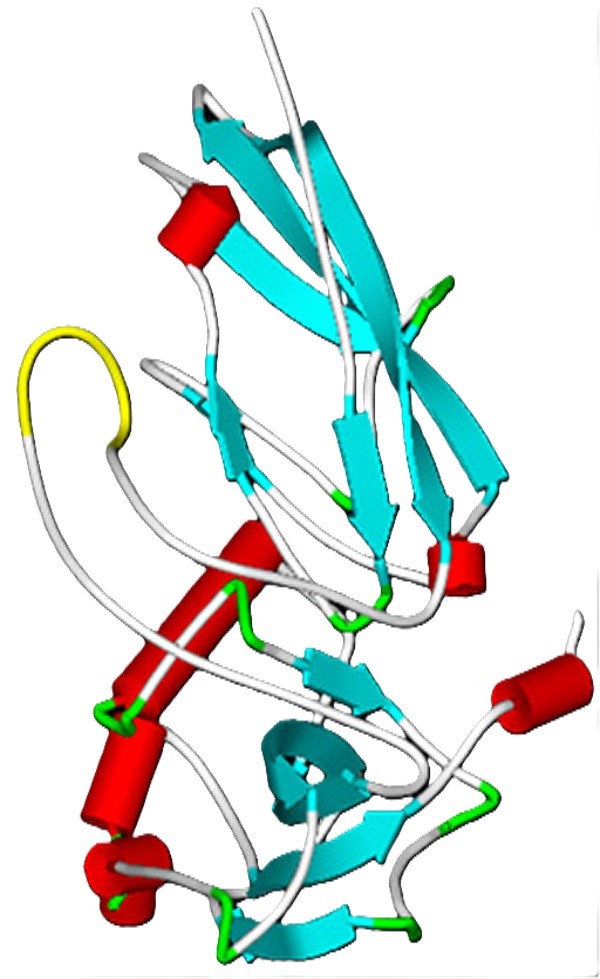 Figure 7. 3D-model of the N-terminus of α-dystroglycan with the myc-tag inserted in position 170. Color code for structural elements: light blue (β-strand), red (α-helix), grey (turn or loop) and yellow (myc tag). (Morlacchi et al., 2012)
Figure 7. 3D-model of the N-terminus of α-dystroglycan with the myc-tag inserted in position 170. Color code for structural elements: light blue (β-strand), red (α-helix), grey (turn or loop) and yellow (myc tag). (Morlacchi et al., 2012)
Comparison of Protein Tags
The choice of a protein tag often depends on the specific needs of the experiment. Each tag provides distinct advantages and disadvantages in terms of its size, ease of use, solubility enhancement, and ability to facilitate protein purification or detection.
| Tag Type | Size | Solubility Enhancement | Purification Method | Cleavage | Use Cases |
| GST-tag | Large (~26 kDa) |
Strong | Glutathione affinity chromatography | Yes | Protein solubility, purification, enzymatic studies |
| His-tag | Small (6-10 residues) |
Moderate | Metal affinity chromatography | Yes | Protein purification, rapid screening, enzymes |
| MBP-tag | Large (~42 kDa) |
Strong | Amylose affinity chromatography | Yes | Protein solubility, large-scale production |
| Flag-tag | Small (~8 residues) |
None | Anti-Flag antibody affinity | Yes | Detection, protein interaction studies |
| SUMO-tag | Medium (~12 kDa) |
Strong | Ni-NTA or His-tag affinity | Yes | Stability, protein-protein interactions |
| c-Myc-tag | Small (~10 residues) |
None | Anti-Myc antibody affinity | Yes | Detection, cancer research, signaling pathways |
Creative Biostructure has extensive experience in tagged protein expression and protein purification. Our dedicated technical support platform is designed to accelerate your project with expert guidance and resources. Explore our related services for more details, and don't hesitate to contact us.
References
- Morlacchi S, Sciandra F, Bigotti MG, et al. Insertion of a myc-tag within α-dystroglycan domains improves its biochemical and microscopic detection. BMC Biochem. 2012;13(1):14.
- Nonaka H, Fujishima S hei, Uchinomiya S hei, Ojida A, Hamachi I. FLAG-tag selective covalent protein labeling via a binding-induced acyl-transfer reaction. Bioorganic & Medicinal Chemistry Letters. 2009;19(23):6696-6699.
- Takahira I, Fuchida H, Tabata S, et al. Design of a binuclear Ni(Ii)–iminodiacetic acid (Ida) complex for selective recognition and covalent labeling of His-tag fused proteins. Bioorganic & Medicinal Chemistry Letters. 2014;24(13):2855-2858.