How to Increase Exosome Production?
In recent years, extracellular vesicles (EVs), especially exosomes, have emerged as a promising strategy for the diagnosis and treatment of a wide range of diseases, such as cancer, and for promoting tissue regeneration in a variety of disorders, including cardiomyopathies and spinal cord diseases. Despite the great potential of exosome-based therapies, their low yield and the inability to scale up production remain a great challenge to overcome for clinical translation.
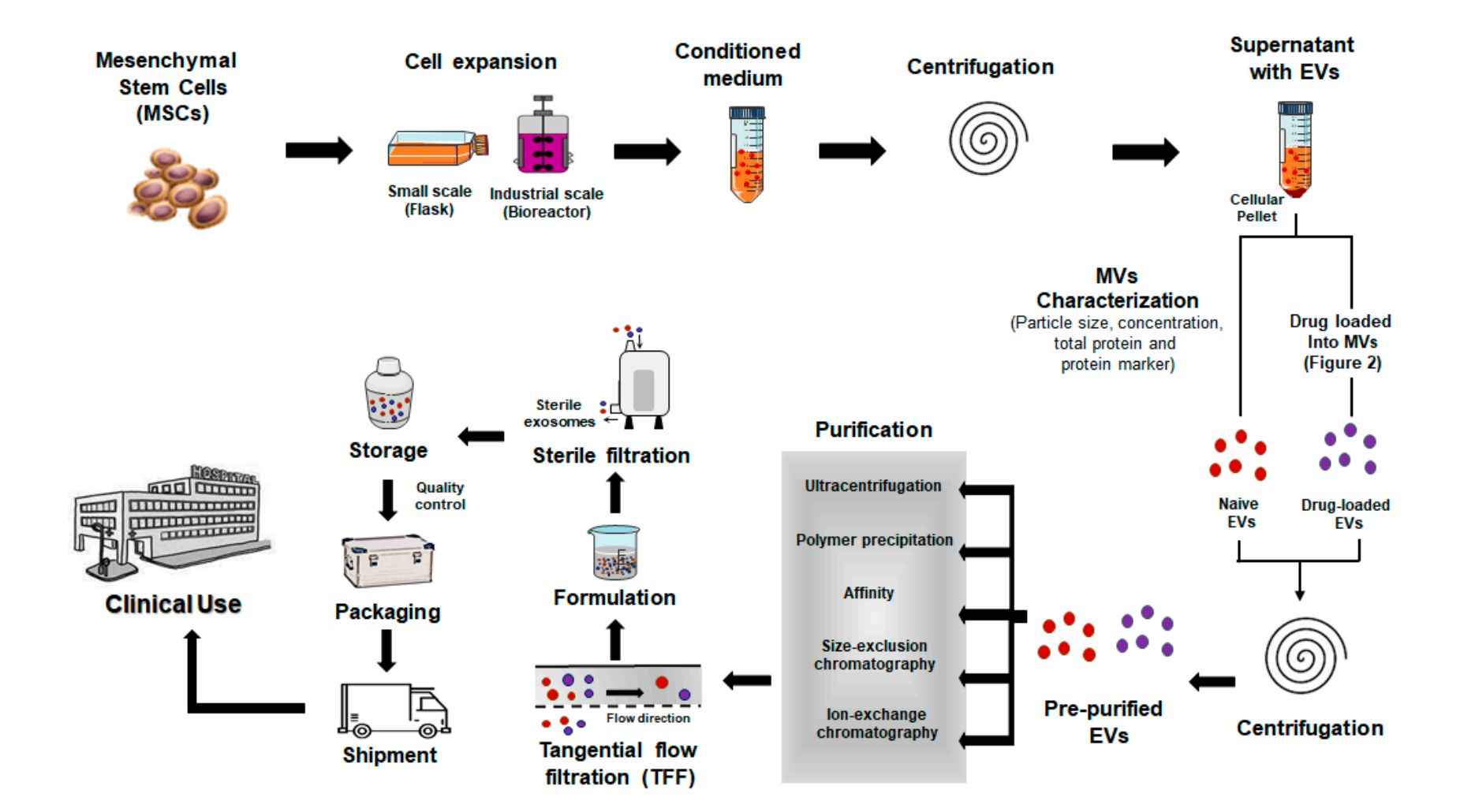 Figure 1. Clinical manufacturing approaches to produce exosome-based therapeutics. (Ortega A, et al., 2020)
Figure 1. Clinical manufacturing approaches to produce exosome-based therapeutics. (Ortega A, et al., 2020)
Why Need to Increase Exosome Production?
Exosome-based therapies offer advantages over cell-based therapies in terms of physicochemical stability, longer blood circulation time, and biocompatibility. However, low yields and scalability issues need to be addressed to achieve industrial-scale production. Traditional exosome production processes are based on expensive two-dimensional cell culture protocols that require large amounts of cell culture plastic, media, and space, which highlights the requirement for more scalable approaches.
What Methods Can Be Used to Increase Exosome Production?
Scientists have developed several strategies to enhance exosome release from cultured cells. Altering the cell culture environment, such as three-dimensional (3D) culture, chemical stimulation, physical stimulation, physiological modifications, and genetic manipulation of the source cells, are the most common ways to increase the quantity and quality of exosomes.
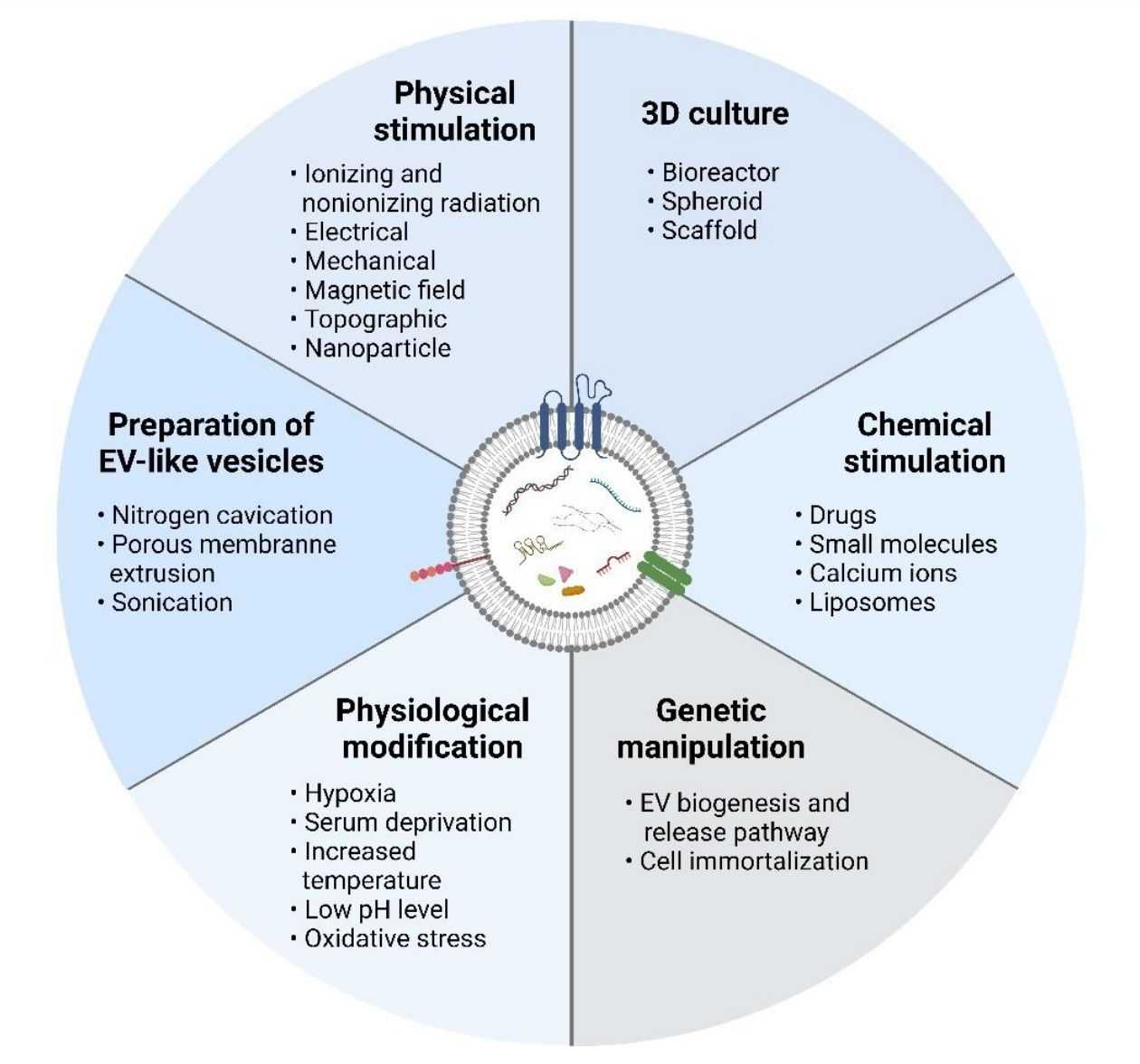 Figure 2. Methods for facilitating exosome scale-up. (Ng CY, et al., 2022)
Figure 2. Methods for facilitating exosome scale-up. (Ng CY, et al., 2022)
Stimulating exosome production by physical signals
- Mechanical loading - Mechanical stimulation may interfere with the cellular exosome secretion mechanism and increase its production under certain loading conditions. Transfer to industrial mass production programs in a scalable manner by relatively simple bioreactor systems.
- Geometry - Traditional exosome production protocols are based on 2D flask cultures, which are inefficient in terms of space, material, and yield. Switching to 3D geometry optimizes physiological culture conditions and leads to higher exosome secretion.
- Acoustic stimulation - Exosome production can be increased by using high-frequency ultrasound (US) illumination.
- Electrical stimulation - Low-level electrotherapy (ET) has been shown to activate intracellular signaling, including Rho-GTPase and endocytosis, which are involved in the exosome formation mechanism.
- Heat shock - Heat shock may cause changes in the folding state of intracellular proteins, thereby activating intracellular stress response pathways and promoting exosome production.
- Shear stimulation - Shear stimulation may increase exosome secretion by activating cellular signaling pathways. It may also lead to the release of more exosomes by affecting the properties of the cell membrane.
Stimulating exosome production by molecular interference
- Inhibits glycolysis and oxidative phosphorylation - The combination of sodium iodoacetate (IAA) and 2,4-dinitrophenol (DNP) induces a reduction in cellular energy charge by blocking oxidative phosphorylation and glycolysis, inducing cellular stress and thus effectively promoting exosome production.
- Endolysosomal trafficking - Exosome biogenesis occurs during endosome maturation. Inhibition of lysosomal function has been described to prevent endosome maturation, leading to the accumulation of intraluminal vesicles within multivesicular bodies.
- Adiponectin - Adiponectin (APN) isolated from serum can enter via the endosomal pathway by binding to T-calmodulin, and the adiponectin/T-calmodulin system subsequently enhances exosome biogenesis and secretion.
Stimulating exosome production by environmental factors
- Hypoxia - In response to hypoxia, cancer cells release exosomes containing pro-angiogenic micro-RNAs and growth factors. Hypoxia can also be used for the production of therapeutic exosomes.
- Acidity - Low PH also stimulates exosome release. Tumor-associated acidity and hypoxia are major determinants of inducing increased exosome release from human cancer cells.
- Starvation or hyperglycemia - Some research suggests that insufficient or excessive glucose can stimulate exosome release.
Stimulating exosome production by external inducers
- Liposomes - Liposomes are known to interact with cell membranes and stimulate cellular activity through endocytosis. Therefore, liposomes interfere with the process of exosome formation and secretion.
- Nanoparticles - Surface-modified nanoparticles can interfere with the endocytosis process to regulate and stimulate exosome secretion.
Increase exosome production by genetic engineering
Genetic engineering techniques can introduce specific genes into cells to increase exosome production. For example, overexpression of genes encoding exosome production can promote their secretion.
Increase Production of Stem Cell-Derived Exosomes
The mechanism and safety of stem cell-derived exosomes make them a promising therapeutic strategy for regenerative medicine. However, limited yields constrain the full elucidation of their functions and clinical applications. To address this issue, scientists have made various attempts to explore upstream and downstream manipulations to increase the yield of exosomes, including factors such as cell source, culture conditions, isolation, and storage.
Strategies to increase stem cell exosome yield can be identified by combining existing approaches. For example, 3D microcarrier-based suspension cultures may increase exosome yield when certain soluble factors or bioactive materials used as bio-microcarriers are used. In addition, due to their relatively controllable composition and clear therapeutic pathways, fully or semi-artificial exosomes may also be tested for mass production.
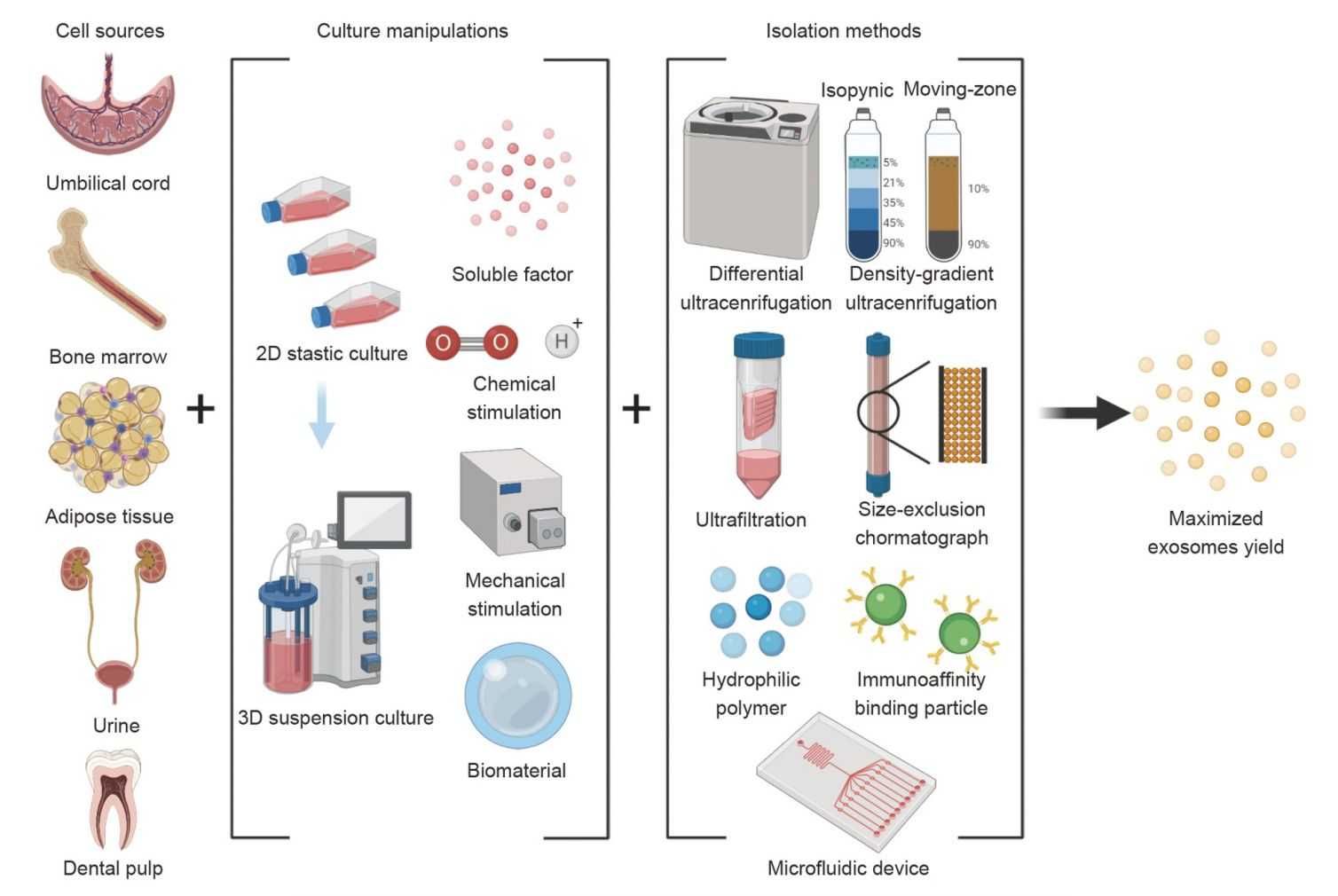 Figure 3. Methods to maximize the yield of stem cell-derived exosomes. (Feng ZY, et al., 2022)
Figure 3. Methods to maximize the yield of stem cell-derived exosomes. (Feng ZY, et al., 2022)
Creative Biostructure offers a range of exosomes isolated from mesenchymal stem cells (MSCs) with low immunogenicity, high biocompatibility, differentiation capacity, and potent immunomodulatory properties, which are valuable tools for clients in regenerative and translational medicine research.
| Cat No. | Product Name | Source |
| Exo-SC03 | HQExo™ Exosome-hTERT | Exosome derived from hTERT-immortalized Mesenchymal Stem Cell |
| Exo-SC04 | HQExo™ Exosome-MSC | Exosome derived from Xeno-Free Human Mesenchymal Stem/Stromal Cells and Media |
| Exo-SC01 | HQExo™ Exosome-PCS-500-011 | Exosome derived from human pre-adipose derived mesenchymal stem cell (PCS-500-011) |
| Exo-SC02-1 | HQExo™ Exosome-PCS-500-012 | Exosome derived from human bone marrow-derived mesenchymal stem cell line (PCS-500-012) |
| Exo-SC02-2 | HQExo™ Exosome-Pla-MSC | Exosome derived from human placental derived mesenchymal stem cell |
| Explore All Exosomes Isolated from Stem Cell Lines | ||
In addition to high-quality, high-purity exosome products, Creative Biostructure offers a one-stop shop for exosome research, including exosome isolation, characterization, analysis, engineering, and application development. If you are interested in our services and products, please contact us by email and describe your specific requirements.
References
- Ortega A, et al. Exosomes as Drug Delivery Systems: Endogenous Nanovehicles for Treatment of Systemic Lupus Erythematosus. Pharmaceutics. 2020. 13(1): 3.
- Ng CY, et al. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. International Journal of Molecular Sciences. 2022. 23(14): 7986.
- Feng ZY, et al. Techniques for increasing the yield of stem cell-derived exosomes: what factors may be involved? Sci China Life Sci. 2022. 65(7): 1325-1341.
- Debbi L, et al. Boosting extracellular vesicle secretion. Biotechnol Adv. 2022. 59: 107983.
