Structural Research of XKR Family of Proteins
XKR family proteins catalyze lipid perturbation. Exposure of the negatively charged lipid phosphatidylserine (PtdSer) to the cell surface catalyzed by XKR is an essential signal for macrophage clearance of apoptotic cells. Although the central role of XKR proteins in apoptosis perturbation and their activation by caspase-3 has been conclusively demonstrated, their molecular structure and how these proteins catalyze lipid transport remain elusive. To address these questions, researchers used cutting-edge cryo-electron microscopy (cryo-EM) to determine the structures of XKR family members.
Structural analysis of XKR9 protein
XKR9 protein is a member of a conserved family associated with apoptotic lipid perturbation. The 43 kDa monomeric membrane protein can be divided into two structurally related repeats, each containing four transmembrane fragments and a helix partially inserted into the lipid bilayer. In the full-length protein, the C-terminus interacts with a hydrophobic pocket located on the inner side of the cell and acts as an inhibitor of protein function. caspase-3 cleaves at a specific site, releasing 16 residues from the C-terminus, thus allowing the pocket to enter the cytoplasm. The XKR9 protein reveals an unknown structure of the XKR family and provides a first insight into its activation by cysteine asparaginase.
Structural research of the human Xkr8-Basigin complex
In addition, the researchers combined cryo-electron microscopy and X-ray crystallography to reveal the overall structure of the Xkr8-Basigin complex, a plasma membrane phospholipid-disrupting enzyme activated by kinases or cysteine asparaginases. The structure shows that the transmembrane region carrying 22 charged amino acids is rectangular and is stabilized by salt bridges between hydrophilic residues in the transmembrane helix. Phosphatidylcholine binding is observed in the hydrophobic cleft of the outer leaflet of the plasma membrane. Six charged residues from top to bottom within the Xkr8 molecule are critical for disrupting phospholipids and provide a pathway for phospholipid translocation. The structure of Xkr8-Basigin provides insight into the molecular mechanism by which XKR family members interfere with phospholipids.
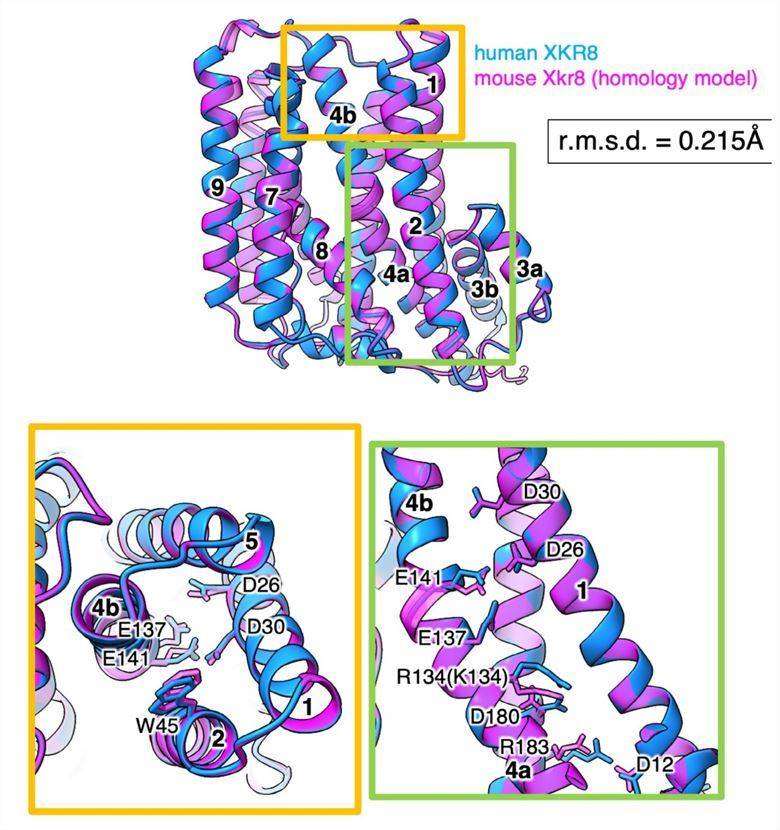 Figure 1. Homology model of the mXkr8 structure. (Sakuragi T, et al., 2021)
Figure 1. Homology model of the mXkr8 structure. (Sakuragi T, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| XKR8-basigin complex bound to Fab fragment | Homo sapiens | Cryo-EM single particle analysis | 3.8 Å | 7DCE |
| Full-length rXKR9 in complex with a sybody | Rattus norvegicus | Cryo-EM single particle analysis | 3.66 Å | 7P14 |
| Caspase-3 cleaved rXKR9 in complex with a sybody | Rattus norvegicus | Cryo-EM single particle analysis | 4.3 Å | 7P16 |
Table 1. Structural research of the XKR family of proteins.
Understanding the structure and function of membrane proteins is essential for developing new drugs and therapies to treat a wide range of diseases. Creative Biostructure is a leading provider of structural analysis services for membrane proteins. Our experts use X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy to determine the three-dimensional structure and understand the function of membrane proteins, including the XKR family of proteins.
Suppose you are interested in gaining a deeper understanding of the structure of membrane proteins and other biomolecules and exploring the possibilities of cutting-edge research in this field. In that case, our team of experts is the right place for you. Our experts have the experience and expertise to provide the best solution for your project. Contact us today to learn more about our comprehensive structural analysis services.
References
- Sakuragi T, et al. The tertiary structure of the human Xkr8-Basigin complex that scrambles phospholipids at plasma membranes. Nat Struct Mol Biol. 2021. 28(10): 825-834.
- Straub MS, et al. Cryo-EM structures of the caspase-activated protein XKR9 involved in apoptotic lipid scrambling. Elife. 2021. 10: e69800.