New Research: Cryo-EM Promotes the Research on UCP1 Working Mechanism
Mitochondria are the main place for cells to carry out aerobic respiration and also the "energy factory" of cell metabolism. Mitochondrial uncoupling protein 1 (UCP1) is highly expressed on the inner mitochondrial membrane and confers on mammalian brown adipose tissue the ability to exclusively burn calories as thermoregulatory heat. Activating brown fat thermogenesis has been reported to be effective against obesity and related metabolic diseases and has clinical application prospects in tumor therapy. UCP1 is the final effector molecule of thermogenesis, and its activity is tightly regulated. Normally, the activity of UCP1 is inhibited by a high concentration of purine nucleotides on the cytoplasmic side (mainly ATP under physiological conditions). When stimulated by cold, sympathetic nerves release norepinephrine, which acts on brown adipocytes to promote fat hydrolysis, and the fatty acids produced can overcome the inhibition of ATP and activate UCP1 to generate heat. In addition, some synthetic chemical small molecule uncouplers, including 2,4-dinitrophenol (DNP), can also effectively activate UCP1.
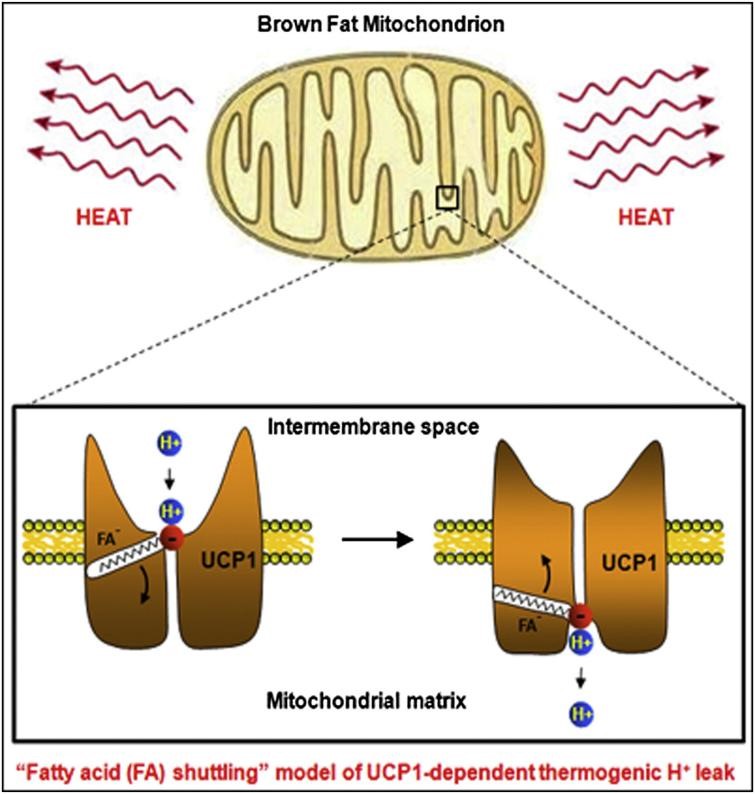 Figure 1. UCP1 working flow model.
Figure 1. UCP1 working flow model.
However, the interpretation of UCP1's mechanism of action has been limited. Since UCP1 is a membrane protein with only 32kDa and no obvious soluble domain, it is difficult to analyze its structure using cryo-electron microscopy. Recently, researchers successfully analyzed the structure of UCP1 in the three states of nucleotide-free binding, DNP binding and ATP binding through the strategy of combining sybody and legobody, with a resolution of 2.51 Å-2.57 Å electron density and built an atomic model. The researchers obtained the sybody that recognizes UCP1 by screening the artificially synthesized nanobody (sybody) library and used the legobody strategy to further increase the molecular weight, in this way, the structure of UCP1 with a molecular weight of only 32kDa can be analyzed by cryo-electron microscopy.
Through structural analysis by cryo-electron microscopy, researchers found that UCP1 in the non-nucleotide binding state is in an open conformation (c-state) on the cytoplasmic side, and the central cavity has more positive charges, which is conducive to binding to the negatively charged purine nucleotides on the cytoplasmic side. UCP1 in the DNP-bound state is also in the c-state, and the overall structure is similar to UCP1 in the non-nucleotide-bound state. The binding site of ATP to UCP1 is similar to that of DNP and is also located in the central cavity of UCP1, where it tightly interacts with multiple TMs.
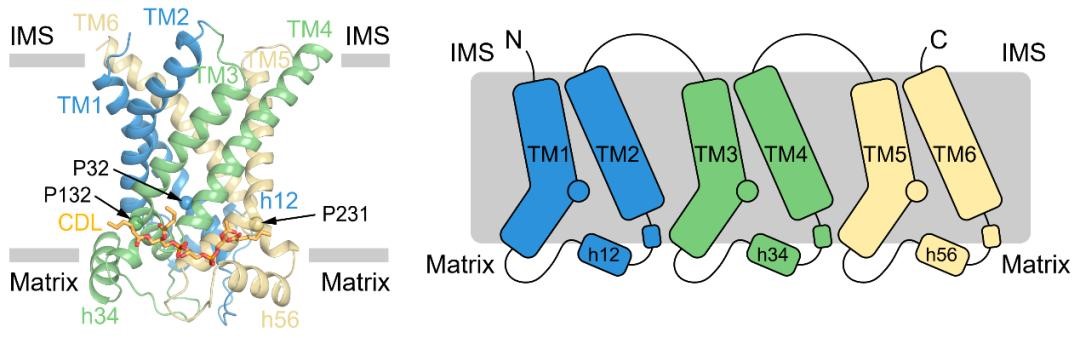 Figure 2. Structure and topology diagram of UCP1.
Figure 2. Structure and topology diagram of UCP1.
This not only provides a structural basis for in-depth understanding of the working mechanism of UCP1, but also develops new ideas for the use of Cryo-EM. Creative Biostructure provides premium cryo-electron microscopy (cryo-EM) service. Please feel free to contact us to discuss your project.
References
- Fedorenko A, Lishko P V, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012, 151(2): 400-413.
- Kang Y, Chen L. Structural basis for the binding of DNP and purine nucleotides onto UCP1. Nature. 2023: 1-2.
- Jones S A., et al. Structural basis of purine nucleotide inhibition of human uncoupling protein 1. Science Advances. 2023, 9(22): eadh4251.