Mempro™ Virus-like Particles (VLPs) Characterization
Characterization and qualification of purified VLPs are crucial factors during VLPs production. Poor characterization can affect both VLPs design and VLPs vaccine development. The functionality of VLPs and the physical characters of VLPs including size and polydispersity are closely related to the efficacy and safety of VLPs vaccine. Creative Biostructure has developed a series comprehensive characterization methods to analyze our VLPs-based products from batch to batch manufacture, assuring to deliver the best services and products, including:
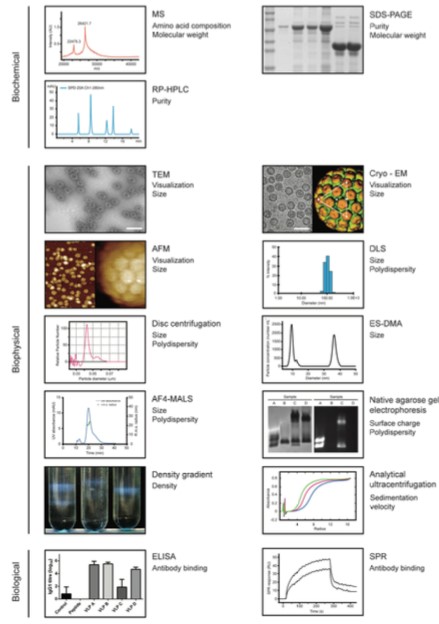
Figure 1. A range of Analytical tools for VLP characterization. (Biotechnology and Bioengineering, 2014)
• SRID for Mempro™ Virus-like Particles (VLPs) Characterization
SRID (Single radial immunodiffusion) is still the only quantification method has been approved by the WHO of HA protein in influenza vaccines. This analytical method offers an estimation of the antigenicity and based on standard antigens. By applying SRID method, the radial diffusion of the viral antigens was measured in an agarose gel which contains specific antibodies. Creative Biostructure has transposed this method to target VLPs vaccines as well.
• HA assay for Mempro™ Virus-like Particles (VLPs) Characterization
The HA (hemagglutination) assay is an analytical method to determine the HA activity in viral preparations. To measure the HA activity, we need to observe the red blood cells (RBCs) agglutination induced by the HA protein. Creative Biostructure has adopted this method in influenza-VLPs production and purification characterization. The main drawbacks of HA assay are susceptibility to false positives and insufficient limit of detection.
• DLS for Mempro™ Virus-like Particles (VLPs) Characterization
It is a challenging task to detect VLPs, while DLS (Dynamic light scattering) is an useful method to analyse the particle size, the aggregation, and the zeta potential of VLPs. DLS, as a non-invasive method, has advantages over traditional microscopy because this method offers the hydrodynamic size of molecules and particles in solution. Creative Biostructure can measure the size of our VLPs in their native state.
• AFFFF-MALS for Mempro™ Virus-like Particles (VLPs) Characterization
AFFFF-MALS (symmetric flow field-flow fractionation using multi-angle light scattering detection) is another technique to separate different particles based on their size. Drying of VLPs may lead to size contraction, AFFFF-MALS is a better analytical method compared with ES-DMA because it measures the VLPs in solution instead of dry measurement.
We provide various Mempro™ Virus-like Particles (VLPs) Characterization services, also including Electron microscopy (TEM), Enzymatic NA activity assay, RP-HPLC, Western blot, LC-MS, ELISA, Surface Plasmon Resonance Immunoassay (SPR), Electrospray differential mobility analysis (ES-DMA), etc. Please feel free to contact us for a detailed quote.
References:
C. M. Thompson, et al. (2013). Analytical technologies for influenza virus-like particle candidate vaccines: challenges and emerging approaches. Virology Journal,10: 141.
S. J. Kaczmarczyk, et al. (2009). Quantitative Characterization of Virus-like Particles by Asymmetrical Flow Field Flow Fractionation, Electrospray Differential Mobility Analysis, and Transmission Electron Microscopy. Biotechnology and Bioengineering, 102(3), 845–855.
L. H. L. Lua, et al. (2014). Bioengineering Virus-Like Particles as Vaccines.Biotechnology and Bioengineering , 111(3): 425-440.