SRID for Mempro™ Virus-like Particles (VLPs) Characterization
During the past decades, Creative Biostructure has established a comprehensive virus-like particles (VLPs) platform which has become a widely applied technology, especially for vaccinology development, faciliating biomedicine research for human health significantly. Creative Biostructure can provide custom VLPs characterization services including SRID (Single Radial Immunodiffusion).
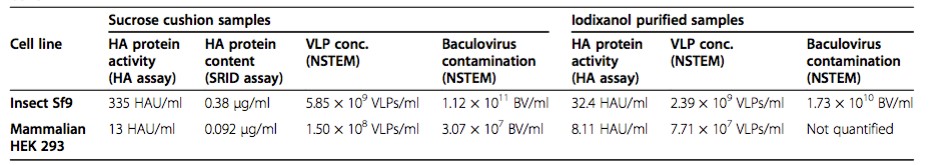 Table 1. Summary of the production performances for the two cell platform, Sf9 insect cells and HEK293SF mammalian cells by using Single Radial Immunodiffusion (SRID) and Hemagglutination Assay (BMC Biotechnology, 2015)
Table 1. Summary of the production performances for the two cell platform, Sf9 insect cells and HEK293SF mammalian cells by using Single Radial Immunodiffusion (SRID) and Hemagglutination Assay (BMC Biotechnology, 2015)
VLPs, as subunit vaccines, are able to be produced in various expression systems containing the native epitopes to stimulate the immune responses efficiently, and they are much more safer than other vaccines thanks to the lack of viral genome. VLP-based influenza vaccines have shown great therapeutic potential in vaccine development. Qualification and characterization of purified VLPs are essential factors for VLPs production. Poor characterization can severely affect VLP-based vaccine development and novel VLPs design.
SRID, which is the standard in vitro potency assay in VLPs characterization field, can specifically detect immunologically active HA protein of influenza virus. SRID is in extensive use of the quantitative estimation of antigens. In this technique, the antigen-antibody precipitation is made to be more sensitive than in double immunodiffusion, because of the incorporation of the antiserum in the agar solution before the gel formation. SRID assay has some limitations as follows: non-aqueous components in vaccines can interfere with the HA antigen diffusion and the requirement of a long processing time.
Creative Biostructure can currently determine the human influenza vaccine doses by the only validated potency test-SRID assay. SRID assay is still the only technique which has been approved by the WHO for the characterization of HA protein in trivalent influenza vaccines. Based on standard antigens, SRID assay can provide an estimation of the antigenicity of the preparation by detecting the radial diffusion of the viral antigens in an agarose gel with specific antibodies. The general detection limitation of SRID assay is approximately 3–5 μg/ml.
Besides SRID assay, Creative Biostructure also provides a wide range of Mempro™ Virus-like Particles (VLPs) Characterization services, such as Dynamic light scattering (DLS), Enzymatic NA activity assay, ELISA, Western blot, Electron microscopy (TEM), LC-MS, Surface Plasmon Resonance Immunoassay (SPR), RP-HPLC, Electrospray differential mobility analysis (ES-DMA), etc. Please feel free to contact us for a detailed quote.
References:
C. M. Thompson, et al. (2015). Critical assessment of influenza VLP production in Sf9 and HEK293 expression systems. BMC Biotechnology, 15: 31.
S. Khurana, et al. (2011). H5N1 Virus-Like Particle Vaccine Elicits Cross-Reactive Neutralizing Antibodies That Preferentially Bind to the Oligomeric Form of Influenza Virus Hemagglutinin in Humans. Journal of Virology, 85(21): 10945-10954.
C. M. Thompson, et al. (2013). Analytical technologies for influenza virus-like particle candidate vaccines: challenges and emerging approaches. Virology Journal,10: 141.