Exosomes Loading Drugs by Incubation
Exosomes are vesicles with a diameter of 30-150nm secreted by living cells and play an essential role in cell-to-cell communication. Due to inherent long-distance communication ability and excellent biocompatibility, exosomes have great potential as drug-delivery vehicles and are particularly suitable for the delivery of therapeutic drugs such as proteins, nucleic acids, and gene therapeutic agents. Exosomes' unique biocompatibility, high stability, and tumor-targeting properties make them of great value in future cancer therapy. There are several methods to successfully encapsulate drugs in exosomes, paving the way for the development of cutting-edge therapeutic strategies. One of the simplest ways to incorporate cargo into exosomes is incubation.
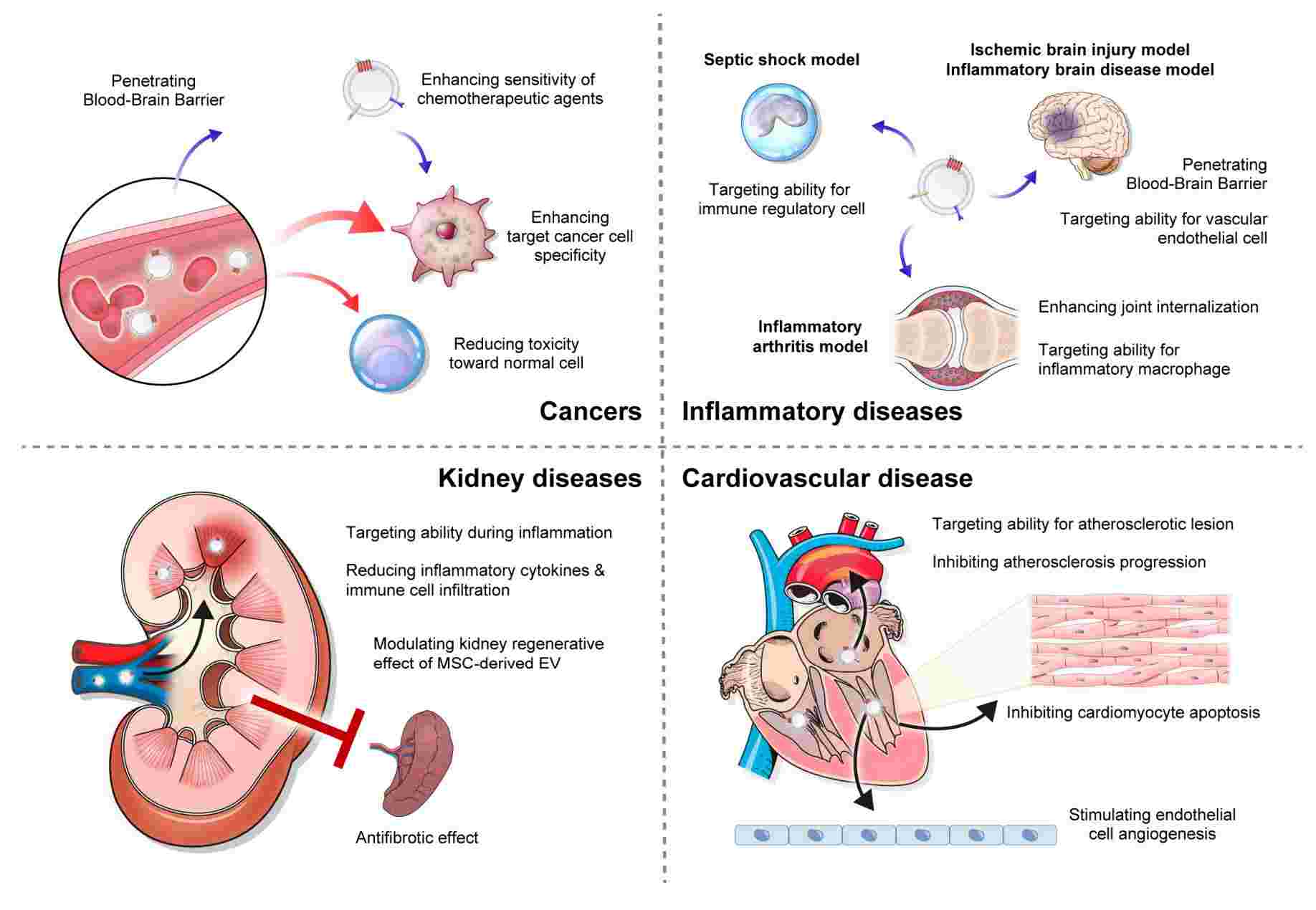 Figure 1. Clinical application of exosome-based drug delivery. (Koh HB, et al., 2023)
Figure 1. Clinical application of exosome-based drug delivery. (Koh HB, et al., 2023)
What is Exosome Drug Loading by Incubation?
Incubation relies on the co-cultivation of exosomes and drug molecules over time. The driving force for loading is the difference in drug concentration inside and outside the exosome membrane. The hydrophobic drug interacts with the lipid layer and the drug diffuses into the exosome lumen along a concentration gradient. The loading efficiency of this method depends on the polarity of the drug molecule, and lipophilic small molecules of low to medium molecular weight, such as catalase, are more readily loaded into exosomes by incubation. The incubation method is simple and does not affect the integrity of the exosome.
 Figure 2. Schematic diagram of exosome and drug incubation. (Xi XM, et al., 2021)
Figure 2. Schematic diagram of exosome and drug incubation. (Xi XM, et al., 2021)
Optimization of the Incubation Method
Since incubation methods have the limitation of low drug loading efficiency, it is usually used in conjunction with other methods to increase exosome loading. A commonly used method is incubation with membrane permeabilizing agents. This method uses membrane permeabilizing surfactants, such as saponins, that result in increased permeability of the exosome membrane. Saponins interact with cholesterol in the cell membrane to promote pore formation in exosomes without compromising the integrity of the lipid bilayer structure. This property of saponins has been used to increase the loading capacity of therapeutic drugs in exosomes over simple incubation methods. In addition, saponins can efficiently load hydrophilic molecules into exosomes, and saponin-assisted methods have shown significant results in active encapsulation techniques.
Application Cases of Exosome Loading by Incubation
Effective targeted delivery of anticancer drugs to triple-negative breast cancer (TNBC) cells remains challenging. Scientists have found that macrophage-derived exosomes loaded with adriamycin (Dox) can target cancer cells and exhibit high anticancer efficacy. Since weakly alkaline conditions promote diffusion of hydrophilic compounds through the lipid bilayer of exosomes, thus optimizing the pH conditions, hydrophilic Dox is loaded into exosomes by incubation. The results showed that the exosome-based formulation exhibited high drug loading, effective accumulation in TNBC cells, and significant inhibition of cancer cell proliferation.
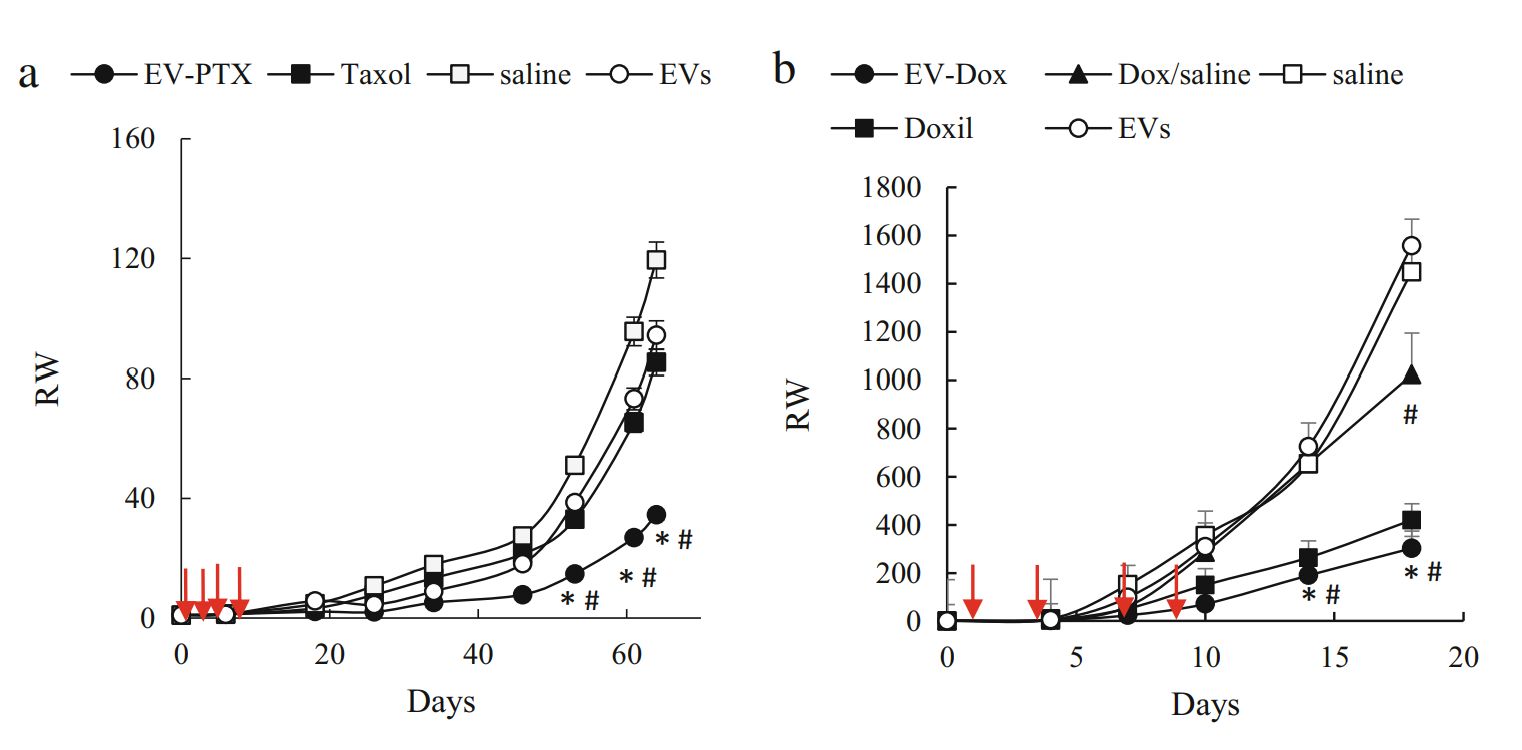 Figure 3. EV-based PTX and Dox suppress tumor growth in orthotopic TNBC mouse models. (Haney MJ, et al., 2020)
Figure 3. EV-based PTX and Dox suppress tumor growth in orthotopic TNBC mouse models. (Haney MJ, et al., 2020)
Exosomes are promising delivery vehicles for therapeutic RNA. The binding of small interfering RNA (siRNA) to cholesterol allows efficient and reproducible loading of therapeutic drugs into exosomes. The researchers coupled cholesterol to siRNA via triethylene glycol (TEG) and 2-aminobutyl-1-3-propanediol (C7) linkers and incubated them with exosomes from umbilical cord mesenchymal stem cells. The results showed that TEG is more suitable for loading exosomes efficiently compared to C7. Hydrophobic modifications can be successfully used as a strategy for the efficient loading of RNA cargo into exosomes.
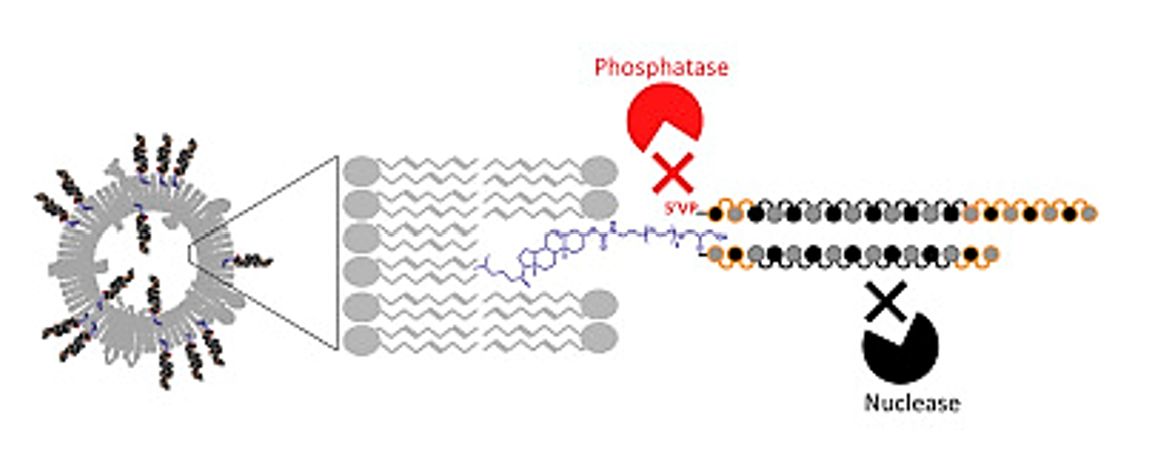 Figure 4. Optimized cholesterol-siRNA chemistry improves productive loading onto extracellular vesicles. (Haraszti RA, et al., 2018)
Figure 4. Optimized cholesterol-siRNA chemistry improves productive loading onto extracellular vesicles. (Haraszti RA, et al., 2018)
Our Services and Products
Choosing the appropriate loading method is an essential factor affecting the use of exosomes as drug carriers. We have been committed to providing diversified exosome services, which are not only limited to exosome isolation, characterization, and biological function analysis. In addition, we also provide exosome kits and high-purity exosome products to help clients explore the clinical applications of exosomes as drug carriers.
An overview of exosome products offered by Creative Biostructure.
| Cat No. | Product Name | Source |
| Exo-CH02 | HQExo™ Exosome-A549 | Exosome derived from human non-small cell lung cancer cell line (A549 cell line) |
| Exo-CH10 | HQExo™ Exosome-HCT116 | Exosome derived from human colorectal carcinoma cell line (HCT116 cell line) |
| Exo-CH16 | HQExo™ Exosome-COLO1 | Exosome derived from human colon carcinoma (COLO1 cell line) |
| Exo-SC02-2 | HQExo™ Exosome-Pla-MSC | Exosome derived from human placental derived mesenchymal stem cell |
| Exo-SC03 | HQExo™ Exosome-hTERT | Exosome derived from hTERT-immortalized Mesenchymal Stem Cell |
| Exo-IC01 | HQExo™ Exosome-BC3 | Exosome derived from human B lymphocyte cell line (BC-3 ) |
| Exo-IC05 | HQExo™ Exosome-JAWSII | Exosome derived from mouse bone marrow immature dendritic cell line (JAWSII) |
| Exo-GC03 | HQExo™ Exosome-GFP | Exosome derived from HEK293 cell line with GFP loaded |
| Exo-GC05 | HQExo™ Exosome-CHO | Exosome derived from Chinese Hamster Ovary cell (CHO cell line) |
| PNE-VA10 | PNExo™ Exosome-Asparagus | Exosome derived from Asparagus |
| PNE-FA18 | PNExo™ Exosome-Apple | Exosome derived from Apple |
| Explore All Exosomes Products | ||
If you are interested in our services and products, please feel free to contact us.
References
- Koh HB, et al. Exosome-Based Drug Delivery: Translation from Bench to Clinic. Pharmaceutics. 2023. 15(8): 2042.
- Xi XM, et al. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021. 76(2): 61-67.
- Haney MJ, et al. Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. J Neuroimmune Pharmacol. 2020. 15(3): 487-500.
- Haraszti RA, et al. Optimized Cholesterol-siRNA Chemistry Improves Productive Loading onto Extracellular Vesicles. Mol Ther. 2018. 26(8): 1973-1982.
- Zeng H, et al. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells. 2023. 12(10): 1416.
