Custom MemPro™ Major Facilitator Superfamily Services
Creative Biostructure provides custom gene-to-structure services for major facilitator superfamily.
Major facilitator superfamily (MFS), also called the uniporter-symporter-antiporter family, is a widespread transport system consist of single-polypeptide secondary carriers which capable only of transporting small solutes in response to chemiosmotic ion gradients. Typical MFS have more than 12 transmembrane segments and they combined with ABC superfamily regulate most uniport-symport-antiport process. MFS has diverse functions including transport of sugars, drugs and Krebs cycle metabolites and it is divided into 17 subfamilies depend on degrees of sequence similarity.
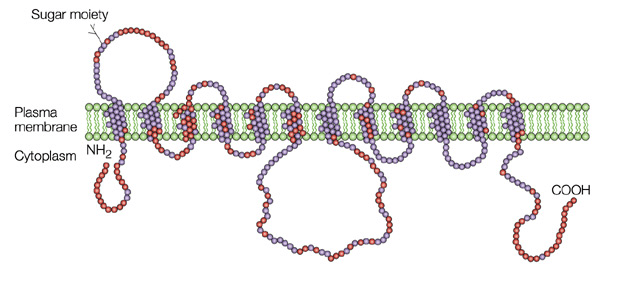 Figure 1. Glucose transporter GLUT4
Figure 1. Glucose transporter GLUT4
Human MFS which mainly responsible for glucose transport is also called glucose transporters (GLUTs). GLUTs have very broad substrate specificity while they can transport both pentoses and hexoses. They are divided into 3 classes: Class I (GLUTs 1-4) are glucose transporters; Class II (GLUTs 5,7,9 and 11) are fructose transporters; and Class III (GLUTs 6,8,10,12 and HMIT1) are structure atypical members of GLUT family which are poorly understand at present.
For now, Class I GLUTs are the best understood MFS receptors.
GLUT1 catalyzes the rate-limiting step in supplying cells of the central nervous system with glucose, an essential fuel for these cells. Additionally, GLUT1 is frequently observed to have abnormal high expression level during oncogenesis in many different tissue types, a process that is probably crucial for tumors to grow beyond a size limited by their glycolytic capacity.
GLUT2 has a uniquely high Km for glucose (∼17 mM), and it is expressed at a very high level in in the basolateral membranes of intestinal and kidney epithelial cells and pancreatic β-cells and of hepatocytes. GLUT2 is also required for the function of glucose sensors present in the hepatoportal vein area and in the central nervous system. These sensors appear to control glucagon secretion, insulin secretion, feeding behavior, and peripheral tissue glucose uptake.
GLUT3 is one of the most expressed receptor which located in both dendrites and axons, and its expression level in different regions of the brain show significant correlations with regional cerebral glucose utilization (rCGU). GLUT3 is also frequently observed in immune cells including lymphocytes, monocytes/macrophages, and platelets. In these cells, rather than directly expressed on the plasma membrane, it is present in intracellular vesicles. When active, they translocate and fuse to the plasma membrane to ensure the glucose uptake and metabolism.
GLUT4 has elusive mechanism of regulation by insulin and the disruption of this regulation in several prevalent insulin-resistant states, including type 2 diabetes mellitus and obesity. Overexpression of GLUT4 expression in skeletal muscle shows insulin sensitivity and glucose tolerance. Conversely, conditional depletion of GLUT4 in either adipose tissue or skeletal muscle causes insulin resistance and a roughly equivalent incidence of diabetic.
A disorder of GLUTs will cause extensive deficiency syndrome called GLUTDS. Additionally, epilepsy and dystonia are also believed to be associated with GLUTs disorder.
References:
Deng D, Sun P, Yan C, et al. Molecular basis of ligand recognition and transport by glucose transporters[J]. Nature, 2015.
Thorens B, Mueckler M. Glucose transporters in the 21st Century[J]. American Journal of Physiology-Endocrinology and Metabolism, 2010, 298(2): E141-E145.