Protein Synthesis
Protein synthesis, a cornerstone of cellular function, drives life at the molecular level. By translating genetic instructions into functional proteins, this process underpins everything from metabolic regulation to the structural integrity of living organisms. This complex process involves two major steps: transcription and translation. These stages work together to convert the genetic code in DNA into functional proteins that are essential for cellular structure, function, and regulation.
In Creative Biostructure, you will learn about the mechanism of protein synthesis and its relevance to protein structure determination. And explore how structural biology techniques such as cryo-electron microscopy (cryo-EM), X-ray crystallography, Nuclear Magnetic Resonance (NMR) spectroscopy, and others allow researchers to unravel the intricacies of protein architecture.
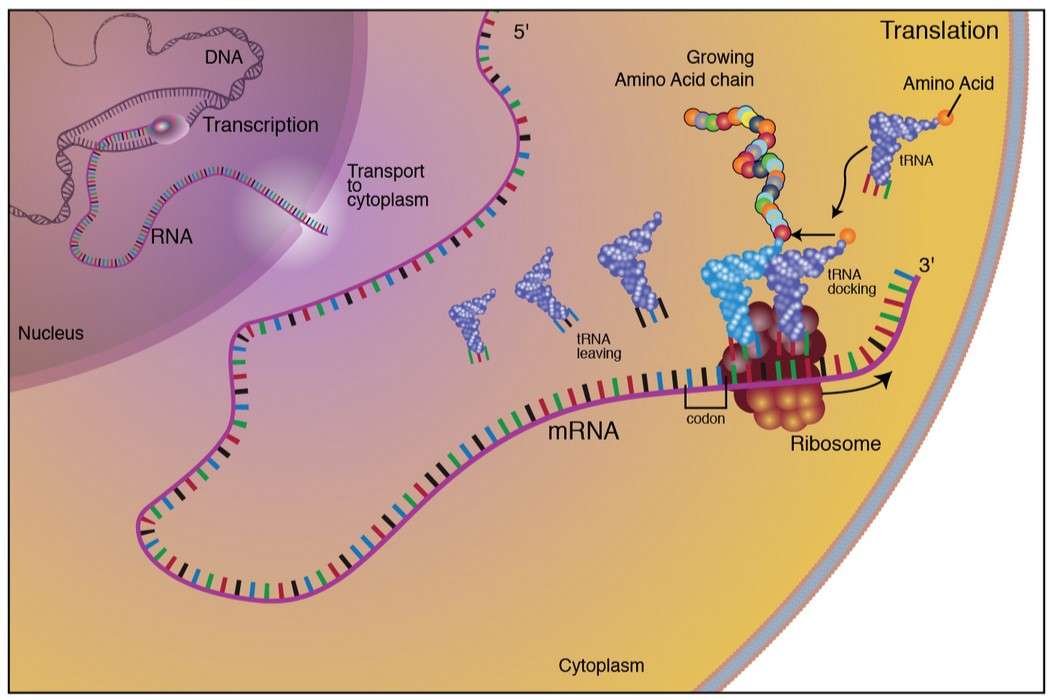 Figure 1. The process of protein synthesis in eukaryotes.
Figure 1. The process of protein synthesis in eukaryotes.
The Molecular Framework of Protein Synthesis
Transcription: Encoding the Blueprint
Transcription, the initial step in protein synthesis, converts DNA sequences into messenger RNA (mRNA). This process begins at the promoter region, where RNA polymerase binds and unwinds the DNA helix. The enzyme synthesizes a complementary RNA strand using ribonucleotides, guided by the DNA template strand. Key elements of transcription include:
- Initiation: The formation of the transcription initiation complex involves general transcription factors, RNA polymerase, and regulatory proteins. The binding of these components to the promoter marks the transcription start site.
- Elongation: RNA polymerase traverses the DNA template and synthesizes the pre-mRNA strand in the 5' to 3' direction. Nucleotide addition is rapid, and proofreading mechanisms ensure fidelity.
- Termination: Specific termination sequences signal the cessation of transcription. The nascent RNA is released, and the DNA helix reanneals.
In eukaryotes, post-transcriptional modifications such as 5' capping, polyadenylation, and splicing refine the pre-mRNA into mature mRNA. These modifications enhance stability, facilitate nuclear export, and ensure accurate translation.
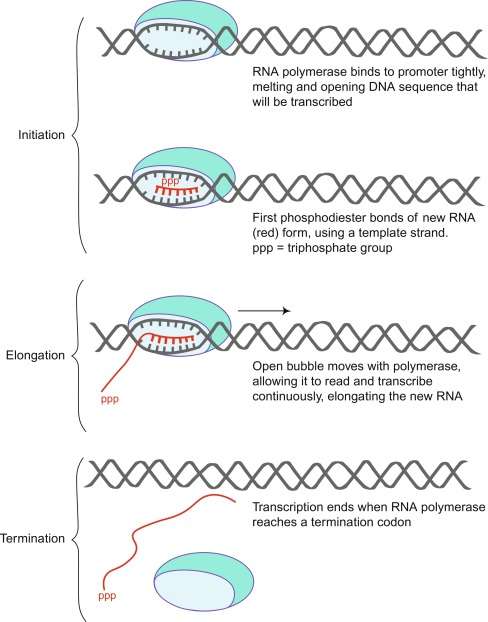 Figure 2. A schematic diagram of RNA polymerase binding to and transcribing DNA to produce new single-stranded RNA. (Shen, 2019)
Figure 2. A schematic diagram of RNA polymerase binding to and transcribing DNA to produce new single-stranded RNA. (Shen, 2019)
Translation: From Nucleotides to Polypeptides
Translation deciphers the mRNA codons into amino acid sequences. This process unfolds in the cytoplasm, where ribosomes, transfer RNA (tRNA), and accessory factors converge. Similar to transcription, the translation process comprises also three sequential phases: initiation, elongation, and termination. Each stage has different protein and RNA molecules that play a role in efficient catalysis. The ribosome also has three main sites: the acceptor site (A site), the peptidyl-transfer site (P site), and the exit site (E site), which accommodate tRNA and facilitate catalysis.
- Initiation: Initiation begins with the 30S subunit to which initiation factor 3 (IF-3) is bound. IF-3 binding prevents premature binding of the 50S subunit and also plays a role in the targeting of the mRNA strand. mRNA binds to this complex using the Shine-Dalgarno sequence. This sequence is a string of 9 nucleotide bases upstream of the AUG start codon on the mRNA. It is complementary to a sequence on the 16S rRNA of the 30S subunit and helps to align the mRNA with the 30S. IF-1 then binds to the A site on the 30S, the site where all charged tRNAs are first bound. IF-1 effectively blocks the premature binding of a tRNA to the A site before the ribosome is fully assembled.
IF-2 delivers the first tRNA to the P site, the site where peptidyl transfer reactions take place. In bacteria, the first tRNA is always an N-formyl modified methionine encoded by an AUG start codon. The formyl group is removed downstream as more amino acids are added to the nascent peptide chain. At this stage, the 30S pre-initiation complex is fully assembled and attracts the 50S subunit for spontaneous assembly. IF-2 is a GTP-binding protein, and hydrolysis of GTP releases all initiation factors from the newly assembled initiation complex. The 70S-mRNA-f-met tRNA complex is now ready for protein synthesis.
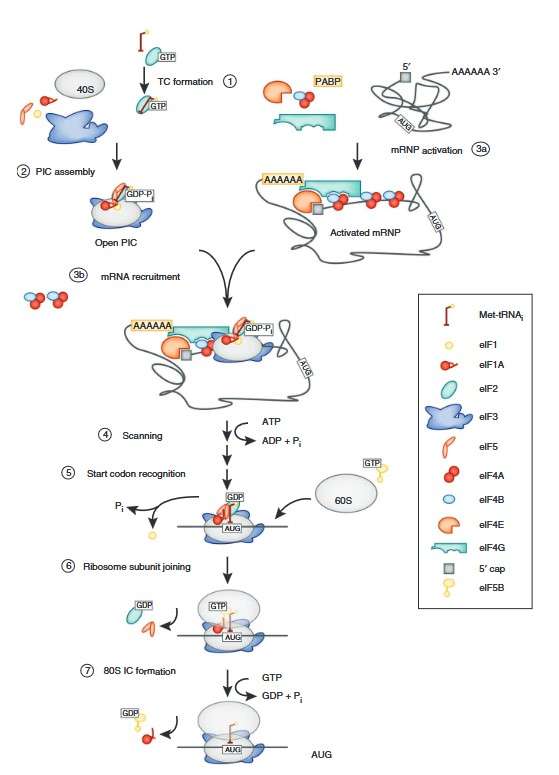 Figure 3. Schematic of the translation initiation pathway in eukaryotes. Initiation begins with the formation of the ternary complex (TC) containing eIF2-GTP and the initiator tRNA (1). The ternary complex is recruited to the 40S subunit with the help of eIFs 1, 1A, 3 and 5 to form the PIC (2). Meanwhile, the mRNA is bound by the eIF4 factors and the PABP to form an activated mRNP (3a), which is then recruited to the PIC (3b). Once bound at the 5′ end of the mRNA, the PIC scans to locate the start (AUG) codon (4). Start codon recognition triggers eIF1 release and conversion of eIF2 to its GDP-bound state, arresting the scanning process (5). eIF2-GDP and eIF5 dissociate, clearing the way for eIF5B to mediate joining of the 60S subunit (6). Subunit joining is followed by GTP hydrolysis by eIF5B and factor dissociation to form the 80S initiation complex (IC) (7). (Aitken and Lorsch, 2012)
Figure 3. Schematic of the translation initiation pathway in eukaryotes. Initiation begins with the formation of the ternary complex (TC) containing eIF2-GTP and the initiator tRNA (1). The ternary complex is recruited to the 40S subunit with the help of eIFs 1, 1A, 3 and 5 to form the PIC (2). Meanwhile, the mRNA is bound by the eIF4 factors and the PABP to form an activated mRNP (3a), which is then recruited to the PIC (3b). Once bound at the 5′ end of the mRNA, the PIC scans to locate the start (AUG) codon (4). Start codon recognition triggers eIF1 release and conversion of eIF2 to its GDP-bound state, arresting the scanning process (5). eIF2-GDP and eIF5 dissociate, clearing the way for eIF5B to mediate joining of the 60S subunit (6). Subunit joining is followed by GTP hydrolysis by eIF5B and factor dissociation to form the 80S initiation complex (IC) (7). (Aitken and Lorsch, 2012)
- Elongation: After the 70S complex has assembled with the initiator tRNA at the P site, the ribosome begins to scan the mRNA sequence. Each codon corresponds to a specific amino acid that is delivered to the ribosome by the elongation factor thermally unstable (EF-Tu). EF-Tu forms a complex with a charged tRNA molecule, places it on the mRNA, and then dissociates from 70S by GTP hydrolysis.
The GTP-bound state of EF-Tu is essential for efficient tRNA delivery, so the cell has evolved a mechanism to recycle EF-Tu by using another protein called elongation factor thermostable (EF-Ts). EF-Ts serves as a guanine nucleotide exchange factor, effectively releasing GDP from EF-Tu so that a new molecule of GTP can be bound. When EF-Tu binds another molecule of GTP, it can again form a tRNA-EF-Tu-GTP complex and continue the tRNA delivery process. Once charged tRNAs are present at both the A and P sites, a peptide bond is formed between the two amino acids by nucleophilic attack of the A site amino acid on the P site amino acid. At this stage, the A site contains the tRNA with the growing peptide chain and the P site has an empty tRNA.
Another GTP-binding protein, elongation factor G (EF-G), catalyzes the movement of tRNAs along the assembly line. This is called translocation, and it frees the A site for further peptidyl transfer reactions. Once EF-G binds to the ribosome, GTP hydrolysis causes the ribosome to undergo a conformational change so that the tRNAs move down from the A and P sites to the P and E sites. The E site is the exit site and empty tRNAs diffuse back into the cytosol where they are reloaded by tRNA synthetases. After EF-G has translocated, the A site is ready to accept a new tRNA. Thus, the elongation cycle continues to provide a growing nascent peptide until a stop codon is encountered.
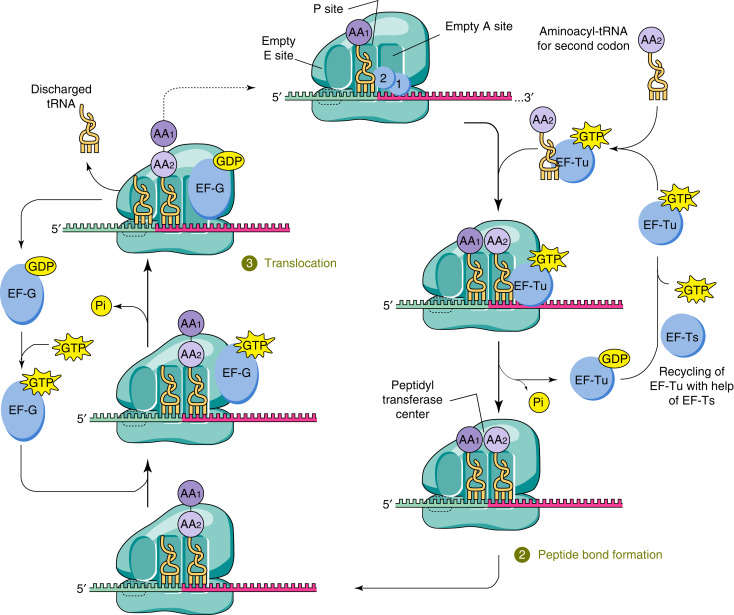 Figure 4. tRNA in translation elongation complex. A cognate aminoacyl-tRNA bound to EF-Tu binds to its cognate codon of the mRNA in the ribosome at the acceptor site (A site), adjacent to the tRNA in the peptidyl site (P site), which is bound to the nascent polypeptide (1). Decoding occurs on the 30S subunit of the ribosome, GTP is hydrolyzed by EF-Tu, which leads to release of aminoacyl-tRNA into the A site if there is correct codon–anticodon pairing. Subsequent peptide bond formation between the nascent polypeptide in the P site and the amino acid attached to the tRNA in the A site results in a peptidyl-tRNA in the A site that is one amino acid longer (2). After translocation of the ribosome, the tRNA attached to the nascent polypeptide is moved to the P site and a new aminoacyl-tRNA is delivered to the empty A site (3). (Doherty and Guo, 2016)
Figure 4. tRNA in translation elongation complex. A cognate aminoacyl-tRNA bound to EF-Tu binds to its cognate codon of the mRNA in the ribosome at the acceptor site (A site), adjacent to the tRNA in the peptidyl site (P site), which is bound to the nascent polypeptide (1). Decoding occurs on the 30S subunit of the ribosome, GTP is hydrolyzed by EF-Tu, which leads to release of aminoacyl-tRNA into the A site if there is correct codon–anticodon pairing. Subsequent peptide bond formation between the nascent polypeptide in the P site and the amino acid attached to the tRNA in the A site results in a peptidyl-tRNA in the A site that is one amino acid longer (2). After translocation of the ribosome, the tRNA attached to the nascent polypeptide is moved to the P site and a new aminoacyl-tRNA is delivered to the empty A site (3). (Doherty and Guo, 2016)
- Termination: Once a stop codon is reached on the mRNA strand, there are no more tRNA molecules to form complementary base pairs with the mRNA. Instead, the release factors 1 and 2 (RF-1/RF-2) recognize the stop codons and bind to the 70S. This triggers hydrolysis of the peptide chain at the P-site and releases the peptide into the cytosol for further processing and folding. RF-3, a GTP-binding protein, binds to the 70S and triggers the release of RF-1/RF-2 by GTP hydrolysis. At this stage, the 70S ribosome has the mRNA and empty tRNA bound. In this state, the 70S cannot carry out protein synthesis and must be recycled. This function is performed by ribosome recycling factor (RRF) and EF-G, which bind to the ribosome and cause its dissociation by GTP hydrolysis. Once the 30S and 50S subunits are free, IF-3 rebinds 30S to prevent premature 70S formation and the initiation cycle can begin again.
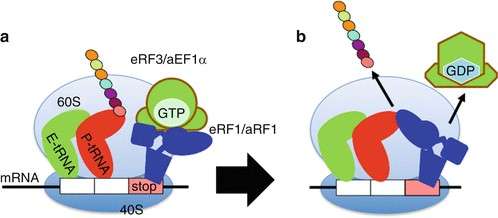 Figure 5. Translation termination. When a stop codon is translocated into the A site, it is recognized by the eukaryotic release factor 1 (eRF1) and associated eRF3 termination factors. eRF1 activates hydrolysis of the ester bond between the completed polypeptide chain and tRNA in the P-site, releasing the polypeptide. (Saito and Ito, 2013)
Figure 5. Translation termination. When a stop codon is translocated into the A site, it is recognized by the eukaryotic release factor 1 (eRF1) and associated eRF3 termination factors. eRF1 activates hydrolysis of the ester bond between the completed polypeptide chain and tRNA in the P-site, releasing the polypeptide. (Saito and Ito, 2013)
Post-Translational Modifications: Refining Functionality
Following synthesis, proteins often undergo post-translational modifications (PTMs) to achieve functional maturity. PTMs include phosphorylation, glycosylation, acetylation, and ubiquitination, among others. These modifications regulate protein activity, localization, and stability, tailoring proteins to specific cellular roles.
Biological Significance of Protein Synthesis
Protein synthesis is indispensable for cellular viability and organismic health. In rapidly dividing cells, efficient synthesis sustains growth and proliferation. In differentiated cells, the process maintains specialized functions. Dysregulation of protein synthesis has been implicated in several diseases, including cancer, neurodegeneration and metabolic disorders.
Moreover, the adaptability of protein synthesis underpins evolutionary innovation. By introducing variability through alternative splicing and codon usage, organisms diversify their proteome, enhancing adaptability and complexity.
Regulation of Protein Synthesis
- Cellular Regulation: Protein synthesis is tightly regulated at the transcriptional, translational and post-translational levels. Regulatory elements such as enhancers and silencers modulate gene expression, while microRNAs and riboswitches influence translation. Ribosome biogenesis and availability also dictate translational capacity, matching protein production to cellular demand.
- Environmental Adaptation: Cells dynamically adjust protein synthesis in response to environmental cues such as nutrient availability, stress, and signaling molecules. For example, during amino acid starvation, the integrated stress response (ISR) modulates translation to conserve resources while prioritizing the production of essential proteins.
Inhibitors of Protein Synthesis
The sheer structural complexity of the ribosome (Ribosome Structure), coupled with its central biological function, makes it a prime target for inhibition. Given the differences between prokaryotic 70S ribosomes and eukaryotic 80S ribosomes, organisms have evolved small molecules that can selectively target 70S ribosomes and 80S ribosomes to selectively kill their target. These inhibitors target almost every stage of protein synthesis, and modern X-ray crystallography has given us a comprehensive understanding of their binding modes and mechanisms of action. Specifically, X-ray crystallography has allowed researchers to visualize the binding sites of various antibiotics on bacterial ribosomes. This understanding has led to the design of drugs that target specific sites involved in protein synthesis, such as the peptidyl transferase center or the tRNA binding sites. It has also been essential in identifying how various inhibitors, such as tetracyclines or macrolides, interact with the ribosome, helping to refine their mechanism of action and improve their selectivity and potency.
Linking Synthesis to Structure
The transition from a linear amino acid chain to a three-dimensional protein is governed by physicochemical forces. Hydrogen bonds, ionic interactions, van der Waals forces, and hydrophobic effects drive protein folding. Misfolding or incomplete synthesis can lead to nonfunctional or toxic aggregates, underscoring the importance of accurate protein synthesis. Structural biology provides powerful methods to visualize proteins at atomic resolution, providing insight into their conformations, dynamics, and interactions. Key techniques include X-ray crystallography, Nuclear Magnetic Resonance (NMR) spectroscopy, and cryo-electron microscopy (cryo-EM).
From Protein Synthesis to Protein Expression Systems
Protein synthesis and protein expression systems are closely related concepts in molecular biology, and both play a critical role in the production of functional proteins. While protein synthesis refers to the cellular process by which ribosomes translate mRNA into polypeptide chains, protein expression systems are the engineered environments or organisms used to produce large quantities of a specific protein for research, therapeutic, or industrial purposes. In essence, protein expression systems use the mechanisms of protein synthesis, such as transcription and translation, to produce recombinant proteins by introducing foreign genes into host cells such as bacteria (Bacterial Expression), yeast (Yeast Expression), or mammalian cells (Mammalian Expression). Understanding the intricacies of protein synthesis is essential for optimizing these expression systems to ensure efficient translation, correct folding, and functional activity of the expressed protein (Protein Function). Thus, advances in protein synthesis mechanisms directly influence the design and refinement of protein expression systems, which are fundamental to biotechnology, drug development, and protein engineering. To explore how these systems are designed and used in practical applications, please visit the following page on Comparison of Protein Expression Systems.
In summary, protein synthesis is a cornerstone of cellular function, translating genetic information into the proteins that sustain life. The complexity of this process, from transcription in the nucleus to translation in the cytoplasm, highlights the sophisticated machinery that cells have evolved to ensure fidelity and efficiency.
At Creative Biostructure, we offer a full range of structural assay services including X-ray crystallography, NMR spectroscopy, cryo-EM and more, providing detailed insights into protein synthesis, structure and function. We also offer protein expression solutions based on protein synthesis, including both intracellular and cell-free systems. Contact us today to find out how our expertise can accelerate your research!
References
- Aitken CE, Lorsch JR. A mechanistic overview of translation initiation in eukaryotes. Nat Struct Mol Biol. 2012;19(6):568-576.
- Doherty J, Guo M. Transfer RNA. In: Encyclopedia of Cell Biology. Elsevier; 2016:309-340.
- Saito K, Ito K. Translation termination. In: Dubitzky W, Wolkenhauer O, Cho KH, Yokota H, eds. Encyclopedia of Systems Biology. Springer; 2013:2271-2275.
- Shen CH. Gene expression: transcription of the genetic code. In: Diagnostic Molecular Biology. Elsevier; 2019:59-86.