Structural Research of Acinetobacter Phage
Acinetobacter is considered an essential pathogen that can cause a wide range of infections, including lung, blood, and urinary tract, and poses a serious clinical challenge due to its increased resistance to antibiotics. In recent years, phage therapy has developed as a promising therapeutic strategy as an alternative to classical antibiotics for the elimination of multi-drug resistant Acinetobacter clinical infections. Scientists have researched the basic structural features of Acinetobacter phages and analyzed the interactions between phages and their hosts, providing promising avenues to address the challenges posed.
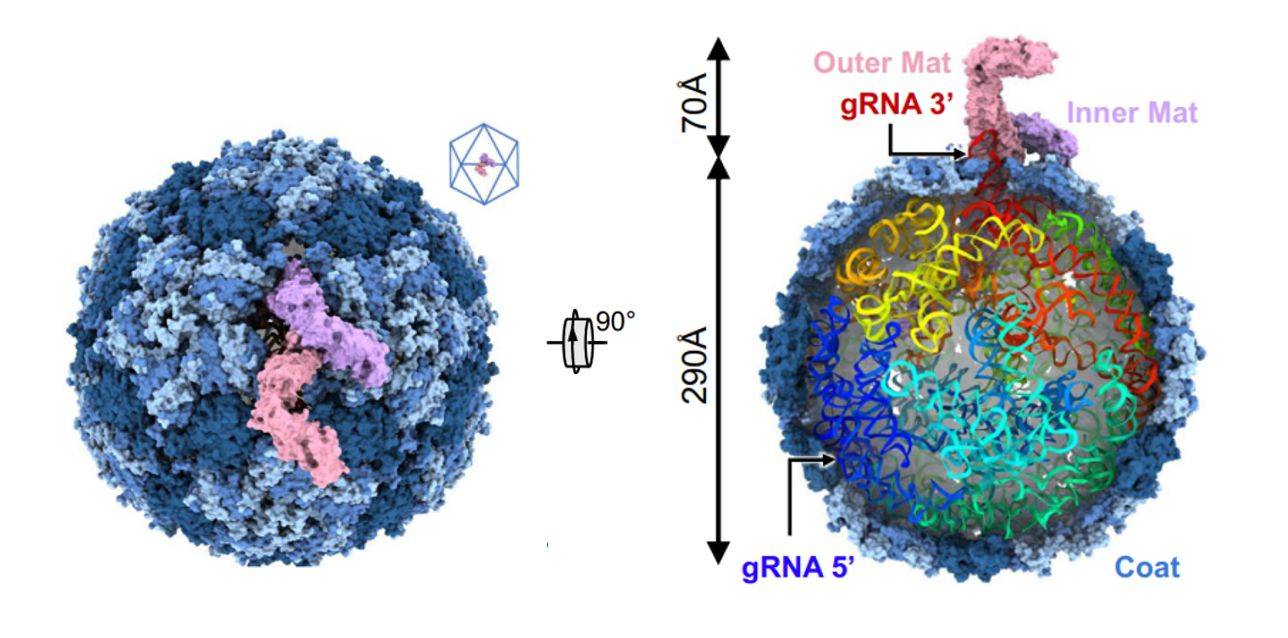 Figure 1. Cryo-EM structure of Acinetobacter phage AP205. (Meng R, et al, 2024)
Figure 1. Cryo-EM structure of Acinetobacter phage AP205. (Meng R, et al, 2024)
Structural Features of Acinetobacter Phage
Typically, the head of a phage exhibits a prismatic shape and contains a single genetic material (DNA/RNA), which is enveloped by proteins. Below the head, there is the neck or collar region that connects to the elongated sheath, also known as the tail. The DNA/RNA is injected into the host cell through the hollow structure of the sheath, surrounded by protective sheath proteins. The base of the sheath features a baseplate to which tail fibers are attached. This baseplate plays a crucial role in host cell attachment and infection, primarily by recognizing the surface receptor of the host cell and facilitating the binding process to complete infection. Tail fiber proteins are typically composed of multiple subunits and exhibit high variability, allowing for the formation of diverse tail fiber structures through different combinations of subunits. This structural diversity enables the infection of various host cells and makes tail fiber proteins useful for the detection of Acinetobacter.
Advances in Structural Research on Acinetobacter Phage
Recently, researchers have delved into the interaction between the Acinetobacter baumannii phage AP205 and type IV bacteriophages. Using cryo-electron microscopy (cryo-EM), the structure of the AP205 virion with asymmetric dimerization of mature proteins was resolved at a resolution of 3.1 Å. The structure shows that 178 Coat copies self-assemble into a nearly icosahedral shell (triangular number T = 3) with a diameter of approximately 290 Å. AP205 has a unique Mat dimer, similar to the shape of the letter "F" at the pseudo-bifold axis of the capsid, and adds 70 Å to the length of the virion. The 3' region of the gRNA is close to the Mat-dimer on the capsid side. Notably, an RNA stem-loop originating from the 3' end of the AP205 gRNA (interacting with the Mat-dimer) is partially exposed outside the capsid. Using these results, an AP205-derived protein scaffold was developed for in situ targeting of type IV bacterial hairs, with research and diagnostic potential.
Acinetobacter infections present a significant clinical challenge, and the rise in drug resistance necessitates the exploration of phage therapy as an alternative to antibiotics. The investigation of the structural aspects of phage-infected hosts holds great potential for the development of diagnostic and therapeutic approaches. Creative Biostructure is a leading provider of viral structure research services and a supplier of virus-like particles (VLPs) products. We offer high-purity Acinetobacter phage VLP (AP205 Proteins) to help clients develop targeted therapeutics.
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Acinetobacter phage AP205 T=4 VLP | Acinetobacter phage AP205 | Cryo-EM single particle analysis | 3.39 Å | 8TW2 |
| Acinetobacter phage AP205 T=3 VLP | Acinetobacter phage AP205 | Cryo-EM single particle analysis | 3 Å | 8TWC |
| Acinetobacter phage AP205 | Acinetobacter phage AP205 | Cryo-EM single particle analysis | 3.11 Å | 8TOC |
| Bacteriophage AP205 coat protein | Acinetobacter phage AP205 | X-ray diffraction | 1.73 Å | 5FS4 |
| Acinetobacter phage 205 (AP205) coat protein in assembled capsid particles | Acinetobacter phage AP205 | SOLID-STATE NMR | / | 5JZR |
| Bacteriophage AP205 virus-like particles | Acinetobacter phage AP205 | Cryo-EM single particle analysis | 6 Å | 5LQP |
Table 1. Structural research of the Acinetobacter phage.
As a leader in viral structure research, Creative Biostructure offers a comprehensive range of services and products to assist clients in developing Acinetobacter diagnostic and therapeutic strategies. Through our cutting-edge expertise in viral structure analysis and possession of advanced instruments such as cryo-electron microscopy (cryo-EM), we can provide invaluable insights into the intricate details of viruses, enabling clients to gain a deeper understanding of their molecular composition and infection mechanisms.
In addition, our high-quality virus-like particle products serve as essential tools for drug discovery and vaccine development, providing a safe and effective platform for accelerating the development of targeted therapies. Please feel free to contact us for more details. With a commitment to scientific excellence and innovation, we are dedicated to supporting our clients in their fight against Acinetobacter infections and improving global health.
References
- Meng R, et al. Structural basis of Acinetobacter type IV pili targeting by an RNA virus. Nat Commun. 2024. 15(1): 2746.
- Tu Q, Pu M, Li Y, et al. Acinetobacter Baumannii Phages: Past, Present and Future. Viruses. 2023. 15(3): 673.