Protein Thermal Shift Assay (PTSA)
Creative Biostructure can provide the most appropriate MagHelix™ PTSA according to customers` requirements. PTSA is a thermo-denaturation assay to measure protein thermal stability. Protein thermal denaturation temperature changes based on different conditions the protein is under. Various approaches used for PTSA include reporter dyes (thermofluor, thiol-specific dyes CPM, rigidity sensitive dyes DCVJ), intrinsic tryptophan fluorescence lifetime, fluorescence wavelengths, static light scattering, Fast Parallel Proteolysis (FastPP), Cellular Thermal Shift Assay (CETSA), Fluorescence-detection Size-Exclusion Chromatography-based ThermoStability assay (FSEC-TS) and Radio ligand Binding Thermo stability Assay (RBTA). PTSA is widely used in drug discovery, protein engineering, structural genomics studies and screening of optimized conditions for protein purification, storage, crystallization, characterization, etc.
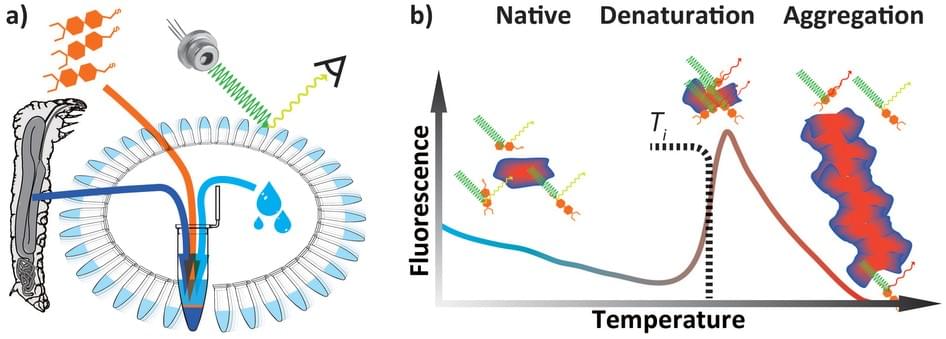 Figure 1. Differential Scanning Fluorimetry (DSF) (Thermal Shift Assay)
Figure 1. Differential Scanning Fluorimetry (DSF) (Thermal Shift Assay)
(a) Sample preparation. (b) Different states of protein and its typical exogenous fluorescence response.
Based on the theory that protein-protein interactions play an important role in many biological processes, to disrupt the interactions can be a strategy to discover new therapeutics. Therefore, accurate quantitative measurement of protein-protein interactions (PPIs) is essential, which can be done by monitoring shifts in thermal stability. Thermal shift assay is a high throughput screening method also called differential scanning fluorimetry (DSF) or the thermofluor assay. Actually, thermofluor assay is just one method for TSA and it is the simplest and most commonly used. It employs reporter dyes to reflect reaction signal and in the assay experiment, a polar environment results in a low fluorescence signal and a nonpolar environment results high fluorescence. Just as shown in the figure, the signal increases when the protein chain begins to unfold and the hydrophobic core is exposed until the protein becomes completely denatured. Moreover, the thermofluor assay with a SYPRO dye can be used in standard quantitative PCR instruments. Except fluorescent, there are also non-fluorescent compounds including thiol-specific dyes CPM. The methods of intrinsic tryptophan fluorescence lifetime and intrinsic tryptophan fluorescence wavelength are based on that intrinsic tryptophan has fluorescence whose lifetime and wavelength are different between unfold and fold protein. Static light scattering monitors the molecule size in different phases, native or denaturation. Fast parallel proteolysis (FastPP) analyzes the thermal melting of protein domains in lysates by using a thermostable protease to cleave at exposed hydrphobic residues. Cellular Thermal Shift Assay (CETSA) refers to thermal shift assays implemented in a cellular format. And in the method of FSEC-TS, the target protein is fused to GFP, heated and treated with a panel of additives, then analyzed by FSEC. The sample can be nanogram to microgram amounts under a variety of conditions. RBTA employs a radiolabeled ligand incubating with the protein. These techniques are available to meet every client`s requirements.
Our MagHelix™ analytical methods for biophysical characterization of biomolecules include but are not limited to:
- Protein Thermal Shift Assay
- Surface Plasmon Resonance
- Isothermal Titration Calorimetry
- Differential Scanning Calorimetry
- Saturation-Transfer Difference
- Bio-Layer Interferometry (BLI) Technology
Ordering Process
References:
Fritz Vollath, et al. Differential scanning fluorimetry provides high throughput data on silk protein transitions. Scientific Reports.2014, Jul 9;4:5625.
Grøftehauge MK, et al. Protein-ligand interactions investigated by thermal shift assay (TSA) and dual polarization interferometry (DPI). Acta Crystallogr D Biol Crystallogr. 2015 Jan 1;71(Pt 1):36-44.
