What Is Cryo-EM Grid Preparation?
Cryo-EM grid preparation is a fundamental technique in structural biology that involves preserving biological samples in a thin layer of vitreous ice for high-resolution electron microscopy analysis. During this process, purified protein samples are applied to specialized grids, rapidly frozen at temperatures below -150°C, and transformed into specimens suitable for imaging.
The preparation involves several crucial steps: applying the sample to an EM grid, removing excess liquid through blotting, and rapidly freezing the remaining thin film to create vitreous ice. This vitrification process is critical as it preserves the sample's native structure while creating conditions suitable for electron microscopic imaging.
Success in cryo-EM grid preparation requires careful control of multiple parameters, including sample concentration, buffer composition, environmental conditions, and freezing speed. The quality of grid preparation directly influences the resolution and reliability of structural data obtained through cryo-EM analysis.
Quick Reference Guide for Cryo-EM Grid Preparation
Success Indicators for Cryo EM Grid Preparation
A comprehensive overview of critical parameters that determine the success of cryo-EM grid preparation.
| Parameter | Optimal Range | Critical Considerations |
| Ice Thickness | 20-100 nm | Optimal thickness depends on sample size |
| Sample Concentration | 0.1-5 mg/mL | Adjust based on molecular weight and complexity |
| Grid Temperature | -180°C (93K) | Rapid cooling essential for vitrification |
| Blotting Time | 2-6 seconds | Sample dependent, optimize for ice thickness |
| Chamber Humidity | 95-100% | Prevents premature sample drying |
| Chamber Temperature | 4-22°C | Match sample storage conditions |
Critical Quality Indicators
- Particle Distribution: Even spacing without aggregation
- Ice Quality: Vitreous without crystallization
- Background: Clean, minimal contamination
- Sample Integrity: No denaturation or degradation
Common Challenges & Solutions Matrix
| Challenge | Symptoms | Solutions | Prevention Strategies |
| Preferred Orientation | Limited particle views | - Add detergents (0.001-0.1%) - Use graphene support films - Try tilted data collection |
- Optimize buffer conditions - Screen multiple grid types |
| Poor Particle Distribution | Empty holes or overcrowding | - Adjust concentration - Modify surface chemistry - Change grid type |
- Pre-test with negative stain - Use different hole sizes |
| Ice Thickness Issues | Too thick/thin ice | - Adjust blotting time - Modify blotting force - Change humidity settings |
- Systematic blotting optimization - Regular grid quality checks |
| Sample Denaturation | Loss of structural integrity | - Reduce exposure time to AWI - Add stabilizing agents - Use continuous support films |
- Optimize sample buffer - Rapid freezing techniques |
Optimization Workflow for Cryo-EM Grid Preparation
1. Sample Preparation Phase
The foundation of successful cryo-EM analysis begins with meticulous sample preparation. This crucial first step involves comprehensive sample characterization using size exclusion chromatography (SEC) and dynamic light scattering (DLS) to ensure sample homogeneity and stability, followed by careful buffer optimization and concentration determination to achieve optimal particle distribution.
2. Grid Selection Strategy
Grid selection represents a critical decision point that significantly impacts the quality of final results. The selection process requires careful consideration of grid material properties (copper, gold, or carbon), appropriate mesh size selection (typically ranging from 200-400), and optimal hole size determination (standard range 1.2-2 µm), along with evaluation of support film requirements based on specific sample characteristics.
3. Initial Screening Process
A systematic screening approach forms the cornerstone of successful cryo-EM grid preparation. This phase encompasses testing multiple preparation conditions while carefully evaluating ice quality and particle distribution patterns. Special attention is given to identifying and addressing any preferred orientation issues that could compromise data quality.
4. Fine-Tuning Optimization
The final optimization phase focuses on refining preparation parameters to achieve the highest quality results. This involves precise adjustment of blotting parameters, careful control of environmental conditions, and implementation of appropriate stabilization strategies to ensure optimal sample preservation and distribution across the grid.
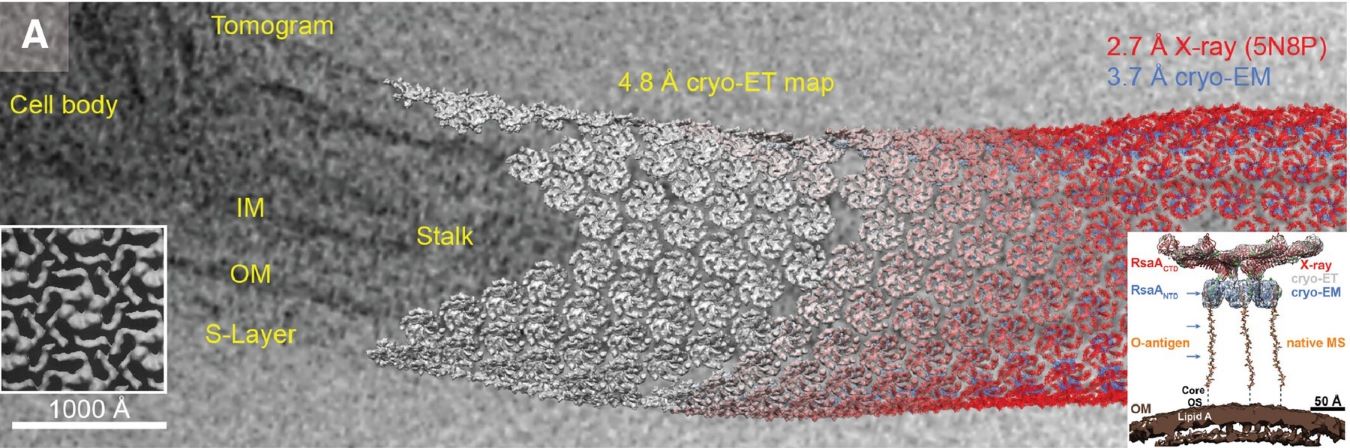
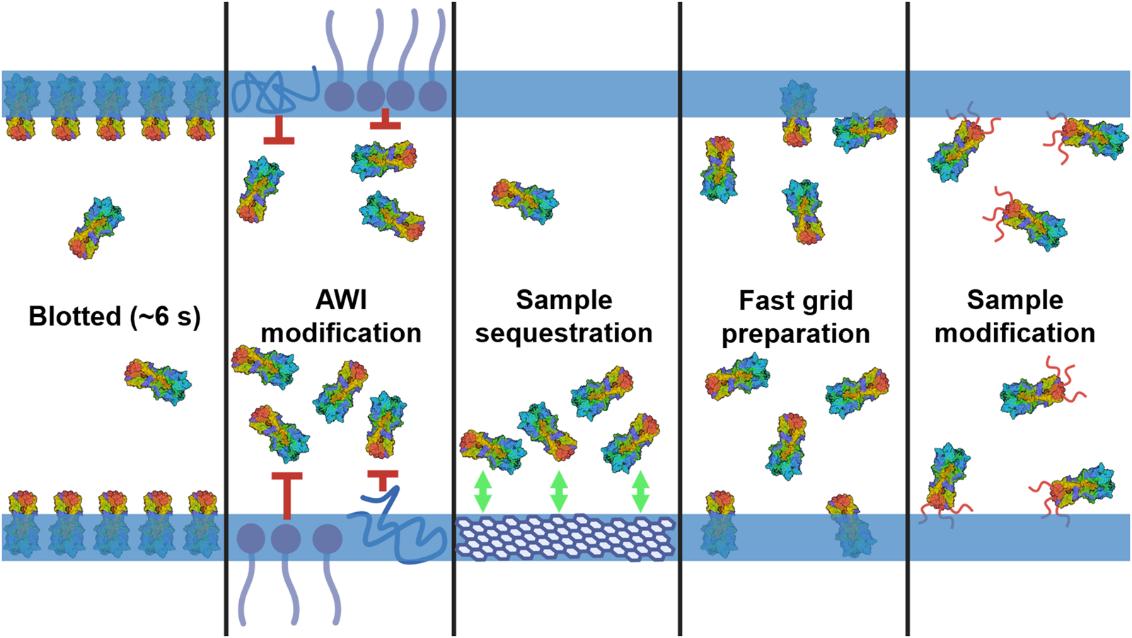 Figure 1. Overview of strategies to address cryo-EM sample preparation challenges. The HA trimer (PDB 7VDF) is shown in a liquid thin film, with the AWI boundaries marked by blue lines. The panels illustrate methods like controlling adsorption, modifying AWI interactions, using support films, speeding up preparation, and altering sample properties to reduce AWI interactions. (Hirst I J, et al., 2024)
Figure 1. Overview of strategies to address cryo-EM sample preparation challenges. The HA trimer (PDB 7VDF) is shown in a liquid thin film, with the AWI boundaries marked by blue lines. The panels illustrate methods like controlling adsorption, modifying AWI interactions, using support films, speeding up preparation, and altering sample properties to reduce AWI interactions. (Hirst I J, et al., 2024)
Equipment-Specific Recommendations
| Equipment | Key Parameters |
| Vitrobot Settings |
|
| Manual Plunger Settings |
|
Quality Control Checkpoints in Cryo-EM Grid Preparation
1. Pre-Grid Preparation Assessment
Before initiating the grid preparation process, a comprehensive pre-preparation quality check is essential. This critical phase involves verifying sample concentration for optimal particle distribution, ensuring buffer conditions are fully optimized for sample stability, and confirming proper grid surface treatment to achieve ideal hydrophilicity for sample application.
2. Real-Time Preparation Monitoring
During the active preparation phase, maintaining strict control over environmental parameters is crucial for success. This involves continuous monitoring of chamber conditions to ensure stability, implementing consistent blotting protocols to achieve uniform ice thickness, and executing rapid sample transfer to liquid ethane to ensure proper vitrification and structural preservation.
3. Post-Preparation Quality Evaluation
The final quality control phase focuses on thorough assessment of the prepared grids to ensure they meet all requirements for high-quality data collection. This evaluation encompasses detailed examination of ice quality and thickness uniformity, comprehensive analysis of particle distribution patterns, and careful screening for potential contamination that could affect imaging quality.
Remember: These parameters serve as starting points and may need adjustment based on specific sample characteristics and requirements.
Understanding the Fundamentals of Cryo EM Grid Preparation
The Physics of Cryo-EM Grid Preparation
- Vitrification Process
The foundation of successful cryo-EM grid preparation lies in understanding the complex physical processes involved. During vitrification, sample molecules suspended in aqueous solution undergo rapid freezing at temperatures below -150°C, transforming the surrounding water into vitreous ice while preserving the native structure of biological specimens. The process involves precise control of critical components, including grid materials and support films, which directly influence sample behavior and image quality.
- Water Dynamics in Grid Preparation
Water behavior plays a pivotal role throughout the preparation process, particularly in the formation of thin films within grid holes. As sample volume decreases during blotting, the confined water molecules create unique physical environments that can significantly impact particle distribution and orientation. The properties of water in these thin films differ markedly from bulk solutions, requiring careful consideration during protocol optimization.
- Understanding the Air-Water Interface
The air-water interface (AWI) represents one of the most critical physical phenomena in grid preparation. As the sample solution thins to form a vitreous ice layer, proteins and other biomolecules may interact with the AWI, potentially leading to conformational changes or preferred orientations. Understanding and managing these interactions is essential for maintaining sample integrity and achieving optimal results.
Sample Requirements and Pre-preparation Considerations
- Concentration Optimization
Sample concentration optimization is crucial, typically ranging from 0.5-3.0 mg/mL depending on molecular weight and complexity. Higher concentrations may be necessary for smaller proteins to achieve adequate particle density, while lower concentrations might be preferred for larger complexes to prevent overcrowding.
- Buffer Selection and Optimization
Buffer composition significantly influences sample stability and behavior during grid preparation. Key considerations include ionic strength, pH stability, and the presence of stabilizing agents. The buffer should maintain sample integrity while being compatible with the vitrification process. Special attention must be paid to components that might affect ice quality or introduce background noise in images.
- Quality Control Implementation
Quality control measures must be implemented at every step of preparation. This includes rigorous sample characterization through analytical techniques such as size-exclusion chromatography and dynamic light scattering to ensure sample homogeneity and stability. Regular monitoring of grid quality and optimization of preparation conditions helps establish reproducible protocols for consistent results.
Advanced Grid Preparation Techniques
Modern Grid Technologies
- Material Selection and Characteristics
Different grid materials offer unique advantages for cryo-EM sample preparation. Gold grids provide superior thermal conductivity and mechanical stability, while copper grids offer cost-effectiveness and reliability. Carbon-based materials excel in creating uniform support films and maintaining sample distribution. Selection depends on specific sample requirements and imaging goals.
- Innovations in Support Films
Recent advances in support film technology have revolutionized grid preparation approaches. Graphene-based supports offer exceptional electron transparency and mechanical strength. Modified support films with functionalized surfaces help control sample orientation and distribution. These innovations particularly benefit challenging samples that require specific surface interactions.
- Emerging Grid Designs
New grid architectures are transforming traditional preparation methods. Enhanced hole patterns optimize ice thickness uniformity, while specialized surface treatments improve sample behavior. Recent developments focus on reducing beam-induced motion and increasing data collection efficiency through improved grid designs.
Sample-Specific Optimization Strategies
- Cryo-EM Grid Optimization for Membrane Proteins
Membrane proteins require specialized approaches due to their unique structural characteristics. Key considerations include detergent selection, lipid environment maintenance, and specific grid surface treatments. Success often depends on careful optimization of these parameters to maintain protein stability and native conformation.
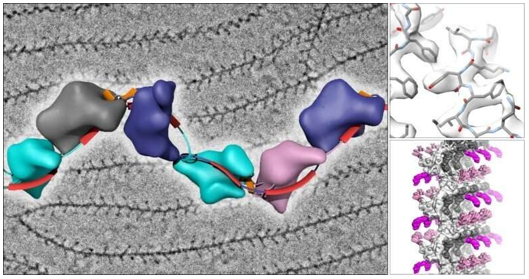
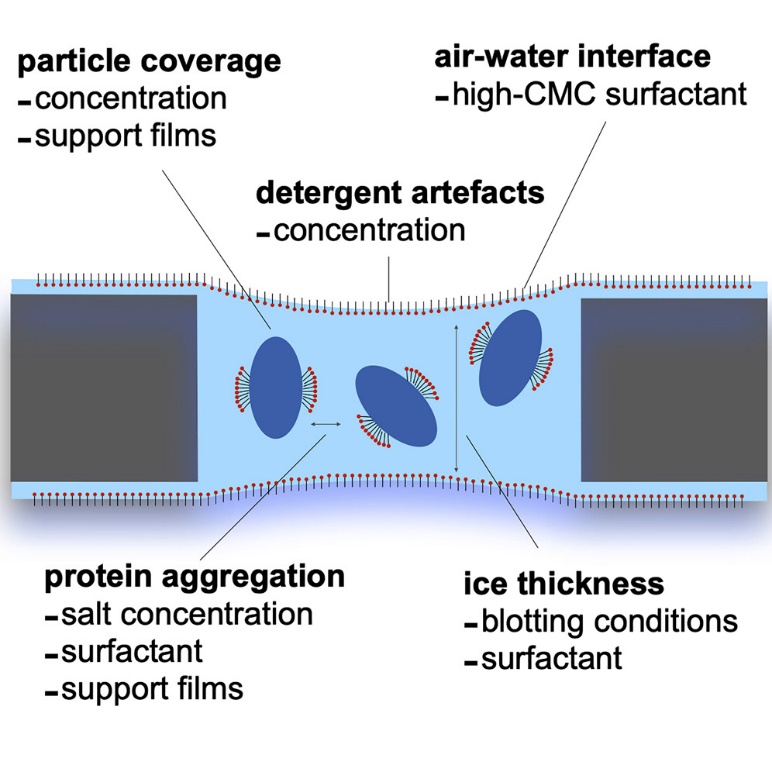 Figure 2. Cryo-EM grid optimization for membrane proteins. (Kampjut D, et al., 2021)
Figure 2. Cryo-EM grid optimization for membrane proteins. (Kampjut D, et al., 2021)
- Large Complex Optimization
Preparing grids for large molecular complexes presents distinct challenges. These samples often require modified blotting conditions and specialized ice thickness control. Optimization focuses on preserving complex integrity while achieving suitable particle distribution for high-quality imaging.
- Small Protein Techniques
Smaller proteins (<150 kDa) demand specific strategies to overcome their size-related challenges. Enhanced contrast methods and modified concentration protocols help improve visibility. Special attention to grid preparation parameters ensures optimal particle density and orientation for successful data collection.
Select Service
- Cryo-EM for Membrane Proteins
- Cryo-EM for Protein Complex
- Cryo-EM for Protein-Ligand Complexes
- Cryo-EM for Small Proteins
- Cryo-EM for Ribosomes
- Cryo-EM for Viral Particle Identification and Characterization
- Cryo-EM for Bacteriophages
- Cryo-EM for DNA Samples
- Cryo-EM Analysis of High-resolution RNA Structures
- RNA Structure Analysis
Troubleshooting & Quality Control
Ice Quality Management
- Mastering Ice Thickness
Ice thickness control represents a critical factor in achieving high-quality cryo-EM data. Proper blotting parameters, environmental humidity control, and grid surface properties all contribute to optimal ice formation. Success depends on maintaining consistent thickness across grid holes while ensuring adequate sample embedding.
- Vitrification Process Optimization
Achieving optimal vitrification requires precise control of freezing conditions. Critical factors include plunge speed, ethane temperature maintenance, and transfer timing. Careful attention to these parameters prevents crystalline ice formation and ensures sample preservation in its native state.
- Effective Contamination Control
Contamination prevention starts with maintaining a clean working environment and proper handling procedures. Essential measures include regular cleaning of preparation tools, careful storage of grids, and monitoring of chamber cleanliness. These steps minimize ice contamination and maximize usable areas for data collection.
Particle Distribution Optimization
- Preventing Sample Aggregation
Sample aggregation often poses significant challenges in cryo-EM preparation. Key preventive measures focus on buffer optimization, temperature control during preparation, and appropriate concentration adjustment. Understanding sample behavior helps develop effective strategies to maintain monodisperse particles.
- Managing Preferred Orientation
Addressing preferred orientation issues requires systematic approach modification. Solutions may include adjusting buffer conditions, incorporating specific additives, or modifying grid surface properties. Success often comes from combining multiple strategies to achieve diverse particle views.
- Concentration Optimization
Finding the optimal sample concentration balance is crucial for high-quality data collection. Too high concentrations lead to overcrowding, while too low concentrations result in insufficient particles for efficient data collection. Careful adjustment based on sample characteristics ensures ideal particle distribution.
In conclusion, successful cryo-EM grid preparation requires careful attention to multiple critical factors, from sample preparation to final quality control. While this guide provides comprehensive insights into the process, each project may present unique challenges requiring specialized expertise.
As a leading provider of structural biology services, Creative Biostructure offers professional cryo-EM services with state-of-the-art facilities and extensive experience in handling diverse sample types. Whether you're working with challenging membrane proteins, large complexes, or small proteins, our expert team is ready to support your research goals. Contact us to discuss your project requirements and discover how our cryo-EM expertise can accelerate your structural biology research.
References
- Sgro G G, Costa T R D. Cryo-EM grid preparation of membrane protein samples for single particle analysis. Frontiers in Molecular Biosciences. 2018, 5: 74.
- Klebl D P, Gravett M S C, Kontziampasis D, et al. Need for speed: examining protein behavior during CryoEM grid preparation at different timescales. Structure. 2020, 28(11): 1238-1248. e4.
- Kampjut D, Steiner J, Sazanov L A. Cryo-EM grid optimization for membrane proteins. Iscience. 2021, 24(3).
- Klebl D P, Kay R W, Sobott F, et al. Towards sub-millisecond cryo-EM grid preparation. Faraday Discussions. 2022, 240: 33-43.
- Hirst I J, Thomas W J R, Davies R A, et al. CryoEM grid preparation: a closer look at advancements and impact of preparation mode and new approaches. Biochemical Society Transactions. 2024: BST20231553.

-1.jpg)