What Is Lipid Nanoparticle
Lipid nanoparticles (LNPs), typically 50-150 nm in diameter, are formed by mixing lipid components to create uniform spheres capable of encapsulating various DNA/RNA payloads. They are simple to synthesize, have scalable and stable manufacturing processes, and offer broad encapsulation capacity, making them widely used non-viral vectors for gene drug delivery. Several LNP-RNA formulations have already been clinically approved.
With their unique physicochemical properties, LNPs enhance nucleic acid stability and bioavailability, prevent rapid degradation in vivo, and enable targeted delivery through particle size, surface modification, and lipid composition adjustments. They also exhibit good biocompatibility and low immunogenicity, making them ideal for non-viral drug delivery systems.
Lipid Nanoparticle Structure and Composition
Composed of synthetic or physiological lipids, LNPs typically include cationic lipids, neutral lipids (e.g., phospholipids), cholesterol, and polyethylene glycol (PEG)-modified lipids. These components form stable nanoparticles through specific chemical interactions, effectively encapsulating and protecting nucleic acids like mRNA from degradation and facilitating cellular uptake.
LNPs are usually composed of four main lipids: cationic lipids, auxiliary lipids, cholesterol and PEG lipids. These lipids have different functions in LNPs:
- Cationic lipids: such as liposomes (DOPE), cationic lipids (such as DMAPE), etc., which encapsulate mRNA and promote its entry into cells through electrostatic adsorption.
- Auxiliary lipids: such as phospholipids (DSPC), cholesterol, etc., provide structural stability and improve the biocompatibility of LNPs.
- PEG lipids: reduce immunogenicity through surface modification and prolong the circulation time of LNPs in the blood.
- Cholesterol: as a structural stabilizer, enhances the mechanical strength and anti-degradation ability of LNPs.
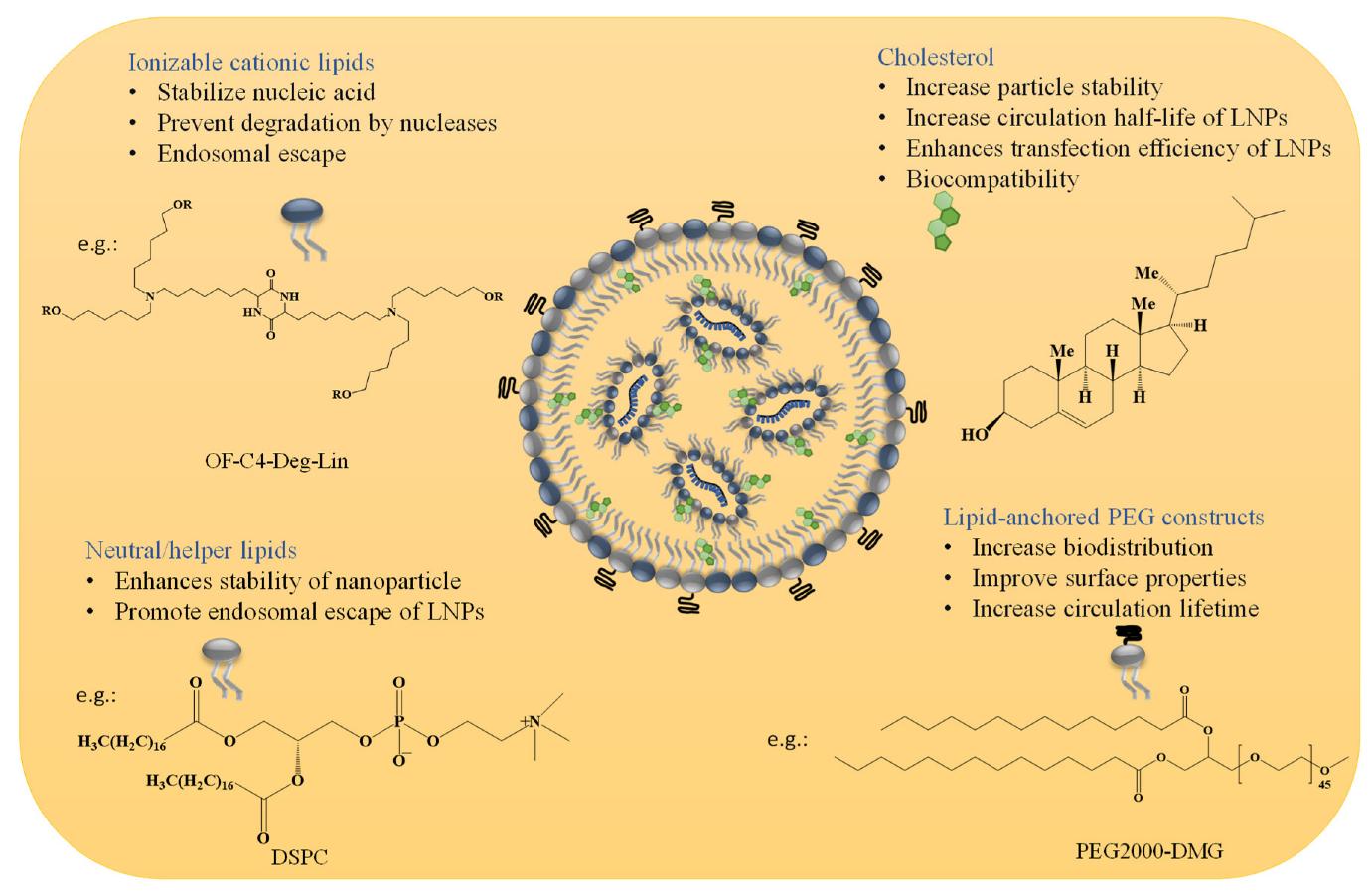 Figure 1. Composition and Functional Roles of LNP Components. (Swetha K, et al., 2023)
Figure 1. Composition and Functional Roles of LNP Components. (Swetha K, et al., 2023)
LNP Preparation and Manufacturing
A. Key Steps in LNP Preparation
The preparation of LNPs usually includes the following steps:
- Lipid dissolution: dissolving lipids in an organic solvent.
- Water phase preparation: adding water phase to the lipid solution to form an initial emulsion by high-pressure homogenization, ultrasonic treatment or emulsification technology.
- Mixing and concentration: after mixing the emulsion, centrifuge or ultrafiltration is performed to remove unreacted lipids to obtain LNP solution.
- Filling and subpackaging: filling the LNP solution into the final container and subpackaging for storage and use.
B. Challenges and Solutions in LNP Manufacturing
| Challenges | Solutions |
|
|
Versatile Applications of Lipid Nanoparticles
LNPs have a wide range of applications, including but not limited to:
- mRNA vaccine delivery: As the core carrier of mRNA vaccines, LNPs can efficiently encapsulate mRNA molecules and deliver them into target cells through endocytosis, thereby inducing immune responses.
- Gene therapy: used to deliver gene editing tools such as siRNA and CRISPR-Cas9 to achieve gene silencing or genome editing.
- Cancer therapy: Enhance tumor treatment effects by targeted delivery of specific drugs or mRNA molecules.
- Other applications: including protein replacement therapy, genome editing, and the development of new nucleic acid drugs.
Select Service/Product
Related Reading
Cryo-EM Advantages in Lipid Nanoparticle Characterization
Cryo-electron microscopy (Cryo-EM) is a cutting-edge imaging technique performed at low temperatures, enabling the acquisition of high-resolution three-dimensional structural information while preventing sample dehydration and artifacts.
Key Advantages of Cryo-EM
- High-Resolution Imaging: Cryo-EM achieves nanoscale to sub-nanoscale resolution, making it ideal for analyzing complex biomolecules and nanoparticles.
- No Chemical Fixation Required: Unlike traditional transmission electron microscopy (TEM), Cryo-EM does not require chemical fixatives, eliminating sample deformation and artifacts.
- Versatile Sample Compatibility: It accommodates a wide range of sample types, including LNPs and protein complexes.
Advantages of Cryo-EM in LNP Characterization
Compared to other techniques, Cryo-EM offers distinct benefits for LNP analysis:
- It is the only method capable of direct, rapid imaging and analysis of lipid-based delivery systems in a near-native state, providing more reliable data.
- Cryo-EM is applicable across various formulations, including different preparation methods, lipid compositions, and storage conditions of liposomes or LNPs.
- It requires only a minimal sample volume, as little as 3 μL.
- A single test delivers multiple critical quality attributes (CQAs), such as morphology, size distribution, active ingredient loading, and encapsulation efficiency. This significantly accelerates the development and optimization of liposome/LNP formulations.
Cryo-EM for LNP Critical Quality Attribute (CQA) Characterization
LNPs are an important component of mRNA vaccines and drug delivery systems. Their CQAs include particle size distribution, morphology, surface charge, etc. The application of Cryo-EM technology in these areas is mainly reflected in the following aspects:
1. Particle Morphology Analysis
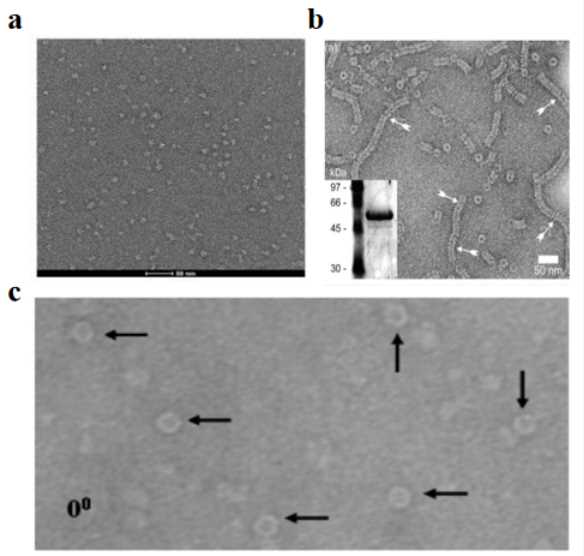
Cryo-EM can directly observe the three-dimensional morphology of LNPs. Studies have found that when the same LNP preparation scheme is used, LNPs loaded with mRNA nucleic acid molecules generally present a vesicle structure, while LNPs loaded with siRNA do not have this feature. This phenomenon reveals that the type of RNA may have a significant impact on the final morphology and function of LNPs. Through cryo-TEM detection technology, the morphology and structural characteristics of LNPs can be intuitively observed.
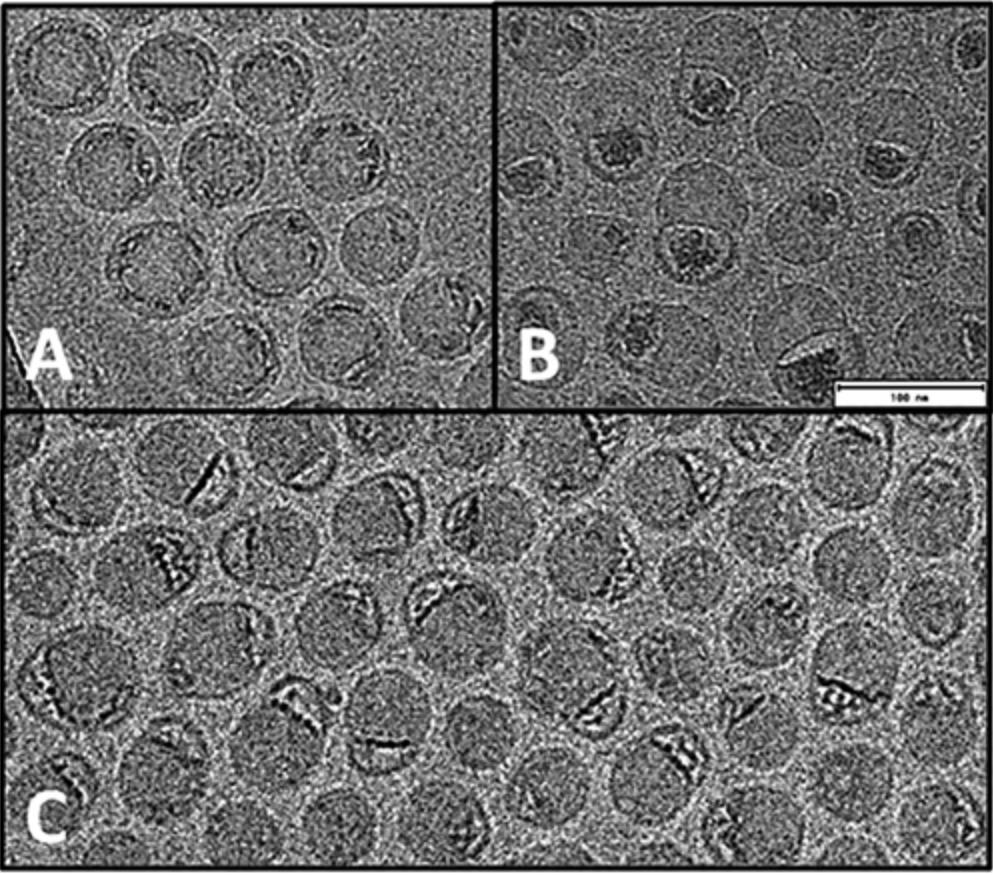 Figure 2. Cryo-EM images of mRNA-LNP preparations reveal diverse morphologies. (A) Highly spherical structure. (B) Nonspherical structure with mRNA in a bleb compartment. (C) Heterogeneous states of mRNA-lipid association between extremes shown in A and B. (Kloczewiak M, et al., 2022)
Figure 2. Cryo-EM images of mRNA-LNP preparations reveal diverse morphologies. (A) Highly spherical structure. (B) Nonspherical structure with mRNA in a bleb compartment. (C) Heterogeneous states of mRNA-lipid association between extremes shown in A and B. (Kloczewiak M, et al., 2022)
2. Particle Size Distribution Analysis
The particle size of lipid nano-delivery carriers significantly influences pharmacokinetics, including systemic circulation, clearance by the mononuclear phagocytic system, tissue extravasation, and intracellular transport. Particle size and distribution affect bioavailability, cellular uptake, and drug delivery efficiency, making them critical quality control parameters for lipid preparations.
Cryo-TEM, combined with dynamic light scattering (DLS), can further validate particle size distribution. While DLS is widely used for liposome and LNP analysis, it measures hydrated particle size and may be influenced by aggregates or scattering at high concentrations. Therefore, structural confirmation methods, such as cryo-TEM, are recommended to ensure accuracy.
Cryo-EM provides particle size distribution results unaffected by particle uniformity and is more applicable for characterizing irregular or non-spherical lipid nanoparticles, including those undergoing dynamic processes like fusion or bubbling.
3. Surface Charge and Chemical Composition
Although Cryo-EM is mainly used for morphological analysis, the surface charge and chemical composition of LNPs can be indirectly inferred by combining with other techniques (such as mass spectrometry). For example, by combining cryo-EM images with mass spectrometry, researchers can identify the modified or functionalized components on the surface of LNPs.
4. Visualization of Active Ingredients
Cryo-TEM can directly confirm whether the active pharmaceutical ingredients are truly encapsulated in liposomes. It can not only visually see the existence of active ingredients in lipid samples, but also further analyze their crystal structure.
5. LNP Encapsulation Efficiency Evaluation
In lipid characterization, the encapsulation rate usually refers to the percentage of the encapsulated substance (such as a drug) in the total amount of the drug in the liposome suspension, which reflects the degree of drug encapsulation by the carrier, but does not reflect whether the drug is encapsulated in the liposome and the proportion of empty shells.
Based on the encapsulation rate, cryo-EM can further reveal the proportion of LNP particles that have not successfully encapsulated nucleic acids. Empty LNP can be considered as a process impurity in the LNP production process. Through the study of empty-shell rate, scientists can more accurately understand the efficiency and problems of nucleic acid encapsulation, so as to optimize and improve the quality of LNP preparation and delivery efficiency.
Under cryo-EM, nucleic acid stains can be used to enhance contrast, directly distinguish the nucleic acids encapsulated inside the LNP and the free nucleic acids outside the LNP, and locate the position of the nucleic acids, so that the empty-shell rate of the LNP can be more accurately and clearly evaluated. By accurately measuring the empty-shell rate, researchers can evaluate the efficiency of the LNP preparation process and optimize it to ensure that as many LNP particles as possible successfully encapsulate nucleic acids, thereby ensuring delivery efficiency, reducing waste and improving effects.
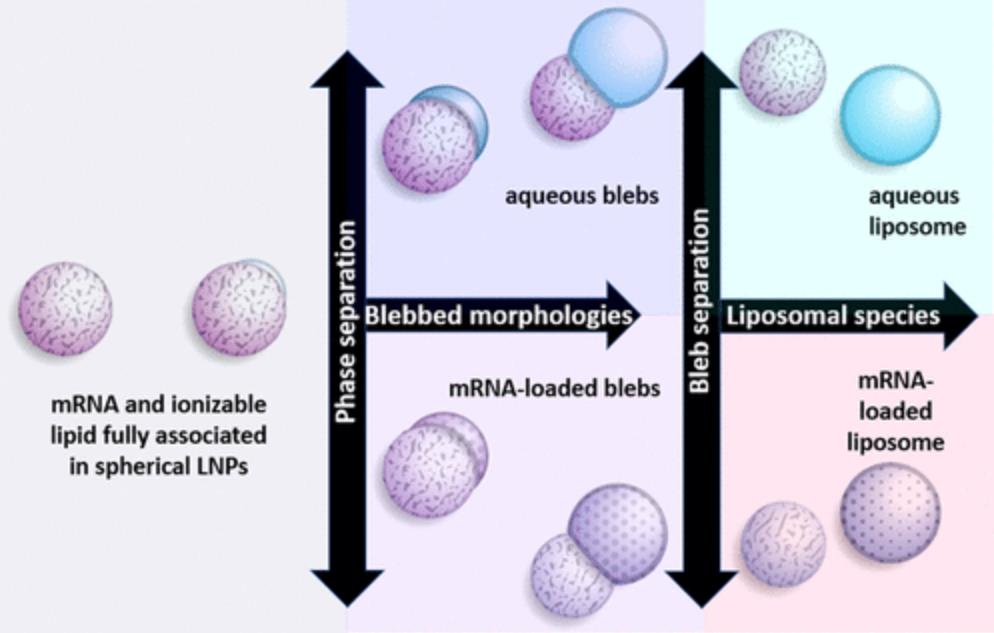 Figure 3. Schematic of mRNA-LNP morphologies: fully associated spherical particles (left), LNPs with bleb compartments enriched in DSPC (center), and dissociated liposomes with or without mRNA (right). (Kloczewiak M, et al., 2022)
Figure 3. Schematic of mRNA-LNP morphologies: fully associated spherical particles (left), LNPs with bleb compartments enriched in DSPC (center), and dissociated liposomes with or without mRNA (right). (Kloczewiak M, et al., 2022)
Cryo-EM Technology for LNP Analysis
A. Sample Preparation and Methodology
- Critical Sample Preparation
Sample preparation represents a crucial step in achieving high-quality cryo-EM imaging of LNPs. The process requires meticulous control of environmental conditions, including humidity and temperature, to preserve the native structure of nanoparticles. The preparation begins with careful application of the sample onto specialized EM grids, followed by precise blotting to achieve optimal sample thickness. The critical vitrification step involves ultra-rapid freezing in liquid ethane, which prevents ice crystal formation and maintains the structural integrity of LNPs.
- Advanced Imaging Protocols
Modern cryo-EM imaging of LNPs employs sophisticated protocols designed to maximize data quality while minimizing sample damage. This involves careful optimization of electron dose, defocus settings, and exposure times. Multiple imaging positions are typically collected per grid to ensure comprehensive sampling and statistical reliability. The implementation of automated data collection strategies enables efficient acquisition of large datasets necessary for detailed structural analysis.
B. Resolution Capabilities
- State-of-the-Art Resolution
Contemporary cryo-EM technology enables visualization of LNP structures at unprecedented resolution levels. The technique can reveal fine structural details including lipid bilayer organization, internal compartmentalization, and cargo distribution. This high-resolution capability is essential for understanding the relationship between LNP structure and function, particularly in the context of drug delivery and vaccine development.
- Advanced Imaging Parameters
The achievement of optimal resolution requires careful control of multiple imaging parameters. This includes precise management of accelerating voltage, beam alignment, and detector settings. Modern cryo-EM systems incorporate sophisticated motion correction algorithms and contrast enhancement techniques to maximize image quality and structural detail.
C. Quality Control Aspects
- System Performance
Maintaining consistent system performance is critical for reliable LNP characterization. This involves regular calibration of microscope components, validation of imaging parameters, and monitoring of system stability. A comprehensive quality control program ensures reproducible results and reliable structural information.
- Sample Quality Management
The assessment of sample quality is fundamental to successful cryo-EM analysis. This includes evaluation of ice quality, sample distribution, and particle integrity. Regular monitoring of these parameters helps maintain consistency across different sample batches and ensures reliable structural characterization.
- Data Validation
A robust data validation process is essential for ensuring the reliability of structural information obtained through cryo-EM. This involves careful assessment of image quality, statistical analysis of particle populations, and verification of structural features. Implementation of standardized validation protocols helps maintain consistency and reliability in LNP characterization.
High-resolution cryo-EM characterization represents a critical tool in understanding and optimizing LNP systems for therapeutic applications. As a leading CRO in structural biology, Creative Biostructure offers cryo-EM service for LNP characterization, supporting your drug delivery system development. Our state-of-the-art facilities and experienced team ensure detailed structural insights into your LNP formulations. Contact us to discuss how our advanced cryo-EM capabilities can accelerate your LNP-based therapeutic development. Our experts are ready to provide customized solutions for your specific characterization needs.
References
- Eygeris Y, Patel S, Jozic A, et al. Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano letters. 2020, 20(6): 4543-4549.
- Hassett K J, Higgins J, Woods A, et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. Journal of Controlled Release. 2021, 335: 237-246.
- Kloczewiak M, Banks J M, Jin L, et al. A biopharmaceutical perspective on higher-order structure and thermal stability of mRNA vaccines. Molecular Pharmaceutics. 2022, 19(7): 2022-2031.
- Cheng M H Y, Leung J, Zhang Y, et al. Induction of bleb structures in lipid nanoparticle formulations of mRNA leads to improved transfection potency. Advanced Materials. 2023, 35(31): 2303370.
- Swetha K, Kotla N G, Tunki L, et al. Recent advances in the lipid nanoparticle-mediated delivery of mRNA vaccines. Vaccines. 2023, 11(3): 658.

-1.jpg)