What Is Recombinant Adeno-Associated Viral Vector
Adeno-associated virus (AAV) is one of the simplest non-enveloped single-stranded DNA defective viruses discovered to date and belongs to the Parvoviridae family. Recombinant adeno-associated viral vectors (rAAV) are derived from non-pathogenic wild-type AAV and are mainly composed of two parts: an icosahedral capsid and a single-stranded genome.
As a non-pathogenic virus capable of long-term gene expression, rAAV has demonstrated extraordinary versatility through its various serotypes, enabling targeted viral delivery to a variety of tissue types. The ability of this vector to effectively transduce both dividing and non-dividing cells, coupled with its minimal immunogenicity, has led to many successful clinical applications. Thanks to its excellent safety, wide range of host cell adaptability, low immunogenicity and the ability to express exogenous genes for a long time in vivo, rAAV has become one of the most widely used vectors in the field of gene drug delivery.
Production Mechanism of AAV Empty Capsids
In the production process of rAAV, the viral genome enters the cell nucleus for replication, and the capsid protein is obtained through transcription and translation and then assembled. Then, under the mediation of the helicase complex, the nucleic acid is guided and assembled into the capsid, and finally assembled into a complete virus particle (full capsid).
However, in actual production, many types of virus particles are produced, such as complete virus (complete genome in the capsid), partially packaged virus (some correct DNA fragments or wrong DNA fragments are packaged), empty virions (no DNA or only some very small DNA fragments inside the capsid), virus aggregates, damaged viruses, etc.
rAAV empty capsid refers to those capsid particles that do not carry complete genomic DNA, and their proportion is usually high. These empty virions are composed of capsid protein, but lack packaging DNA, so they do not have the function of gene delivery.
The formation of empty capsids is mainly closely related to the production process of rAAV vectors. During the assembly process of rAAV, due to the low efficiency of gene packaging, some of the coat proteins may not bind to the DNA, resulting in the formation of empty capsids. In addition, factors such as plasmid ratios, cell culture level, and harvest time also affect the rate of empty capsid.
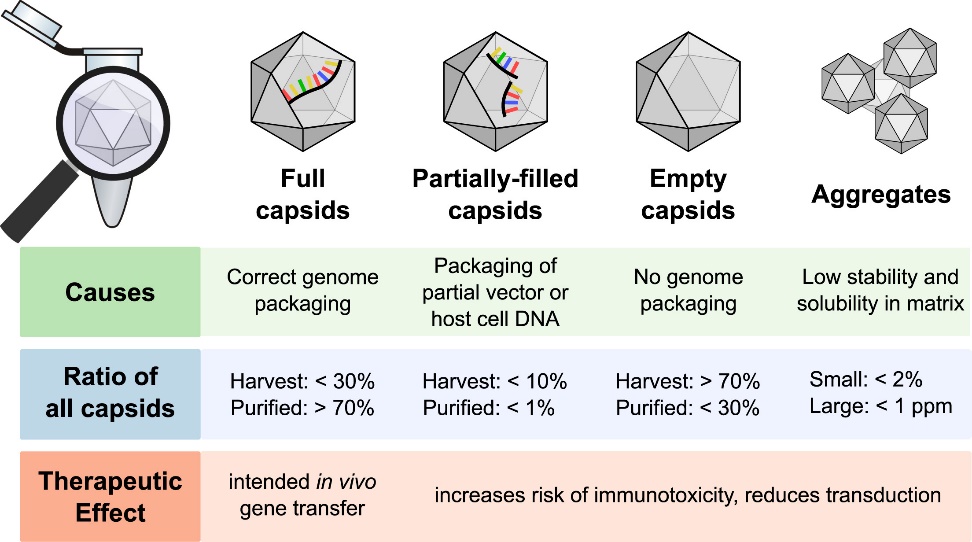 Figure 1. Overview of Capsid Types in rAAV Production. (Gimpel A L, et al., 2021)
Figure 1. Overview of Capsid Types in rAAV Production. (Gimpel A L, et al., 2021)
Importance of Empty-Full AAV Capsid Ratio
The empty-to-full capsid ratio is a critical quality attribute (CQA) in AAV-based gene therapy products. Accurate determination of this ratio is essential for ensuring both the safety and efficacy of the product. While empty capsids inevitably form during AAV production, an excessive proportion of empty capsids can lead to several issues:
- Immune Responses: Empty capsids may trigger immune reactions, which can interfere with the therapeutic effect of the treatment.
- Increased Dose Requirements: Since empty capsids cannot deliver therapeutic genes, a higher dose of the product may be needed to ensure adequate therapeutic payload reaches target cells.
- Reduced Transduction Efficiency: Empty capsids may compete for binding sites on target cells, thus diminishing the transduction efficiency of the full, therapeutic capsids.
- Potential Adverse Events: Empty capsids may disrupt the intended gene therapy effects and, in some cases, contribute to adverse reactions.
Maintaining an optimal empty-to-full capsid ratio is therefore essential for maximizing the therapeutic benefits of AAV-based gene therapies.
Common Methods for Assessing AAV Empty-to-Full Capsid Ratio
Accurate and rapid determination of the empty-to-full capsid ratio is essential for ensuring the efficacy and safety of AAV vector products. Several methods are commonly used for this analysis:
Transmission Electron Microscopy (TEM)
TEM is a direct method for observing AAV particle morphology, enabling the calculation of the empty-to-full capsid ratio based on particle distribution and proportions. This technique offers high resolution but requires specialized equipment.
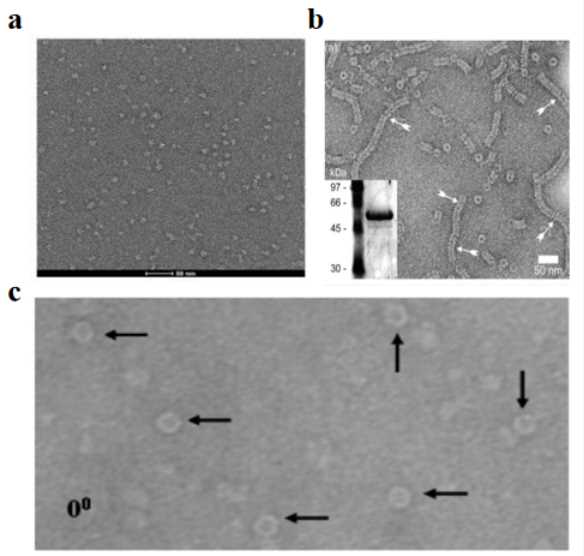
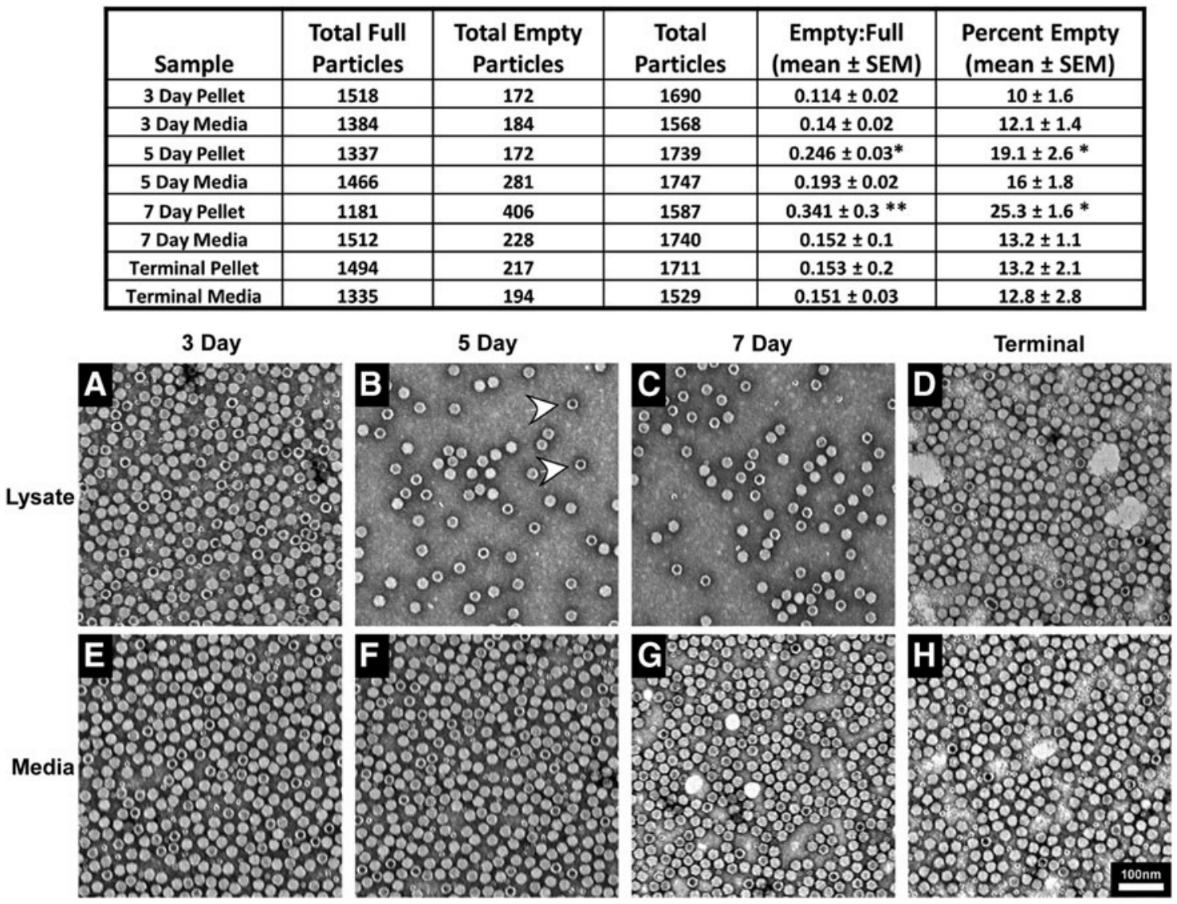 Figure 2. Ratio of Empty to Full AAV Particles from Cellular Lysates and Medium. TEM images of AAV samples from cellular lysates and medium at 3, 5, and 7 days post-transfection. Empty particles (arrowhead in B) are identified by their electron-dense core. The ratio of empty to full particles was quantified from random electron micrographs. (Benskey M J, et al., 2016)
Figure 2. Ratio of Empty to Full AAV Particles from Cellular Lysates and Medium. TEM images of AAV samples from cellular lysates and medium at 3, 5, and 7 days post-transfection. Empty particles (arrowhead in B) are identified by their electron-dense core. The ratio of empty to full particles was quantified from random electron micrographs. (Benskey M J, et al., 2016)
Select Service
Related Reading
Analytical Ultracentrifugation (AUC)
AUC separates different types of AAV particles based on differences in sedimentation velocity. Empty and full capsids exhibit distinct sedimentation coefficients (S-values) due to differences in mass. AUC can accurately separate and quantify empty, partially filled, and full capsids, but it requires large sample volumes and complex procedures.
Ion Exchange Chromatography (AEX)
Ion exchange chromatography separates AAV capsids based on differences in their charge. By selecting appropriate buffers and ion-exchange resins, effective separation of empty and full capsids can be achieved. This method offers good reproducibility and accuracy but is sensitive to buffer selection and may be affected by the presence of other impurities.
Mass Spectrometry (Native MS)
Native mass spectrometry is a rapid, sample-preparation-free technique that assesses the empty-to-full capsid ratio by analyzing the molecular weight distribution of AAV capsid proteins. This method provides high sensitivity and accuracy but requires mass spectrometry equipment and is sensitive to sample complexity.
Sedimentation Velocity Analytical Ultracentrifugation (SV-AUC)
SV-AUC, a variation of AUC, quantifies the empty-to-full capsid ratio by measuring sedimentation velocity. This technique provides high accuracy and reliability but, like AUC, demands large sample volumes and specific experimental conditions.
Capillary Isoelectric Focusing (cIEF)
Capillary isoelectric focusing separates AAV capsids based on differences in their isoelectric points. Empty capsids typically have a lower isoelectric point, allowing their separation by adjusting buffer conditions. This method is simple, fast, and suitable for quality control (QC) testing.
High-Performance Liquid Chromatography (HPLC)
HPLC, often combined with ion exchange or affinity chromatography, allows high-resolution separation and quantification of AAV capsids. This technique offers high sensitivity and accuracy but requires optimized chromatographic conditions to minimize interference from impurities.
Among these, AUC and Cryo-TEM are the dominant methods for assessing the empty capsid ratio in rAAV vectors. Other techniques, such as ddPCR-ELISA and size-exclusion chromatography multi-angle light scattering (SEC-MALS), are also utilized.
Detailed Analysis of AUC Limitations
AUC separates particles based on differences in their sedimentation coefficients. The sedimentation velocity of full and partially filled AAV particles is higher than that of empty capsids, enabling distinction between these types. AUC can quantify empty, full, and partially filled particles, and also detect aggregates and vector fragments. Despite its power, AUC has several limitations:
- Sedimentation Coefficient Limitations: The sedimentation coefficient is a particle property but not an absolute identifier, as particles of different types (e.g., damaged full particles or aggregates) may have similar sedimentation velocities.
- Sample Requirements: AUC requires high-concentration, purified samples (5x10^12 VP/mL) and large sample volumes (500 µL).
- Structural Integrity Risks: Prolonged centrifugation may induce mechanical shear forces that can deform or damage viral particles, affecting the results.
- Inability to Differentiate Aggregates: AUC cannot distinguish between empty and full particles within viral aggregates or identify specific types of damaged particles.
- Low Throughput: AUC runs take approximately 6 hours, with data analysis extending the total time for each batch of 3–7 samples.
- Risk in GMP Environments: In Good Manufacturing Practice (GMP) settings, the potential aerosol risk during centrifugation must be carefully evaluated.
Given these considerations, selecting the appropriate method for analyzing the empty-to-full capsid ratio depends on the specific requirements for accuracy, sample availability, and throughput in quality control settings.
Cryo-EM for rAAV Vector Multi-Attribute Characterization
The successful development and manufacturing of rAAV-based therapeutics critically depend on comprehensive characterization methods to ensure product quality and safety. Traditional analytical approaches face significant limitations in providing detailed insights into critical quality attributes such as capsid composition, genome packaging efficiency, and structural integrity. These challenges have created an urgent need for advanced characterization techniques that can offer higher resolution, better accuracy, and more comprehensive analysis of rAAV vectors throughout the development and manufacturing process.
High-Resolution Imaging in Near-Native State
Cryo-EM is the only technique that allows for the intuitive and rapid imaging of rAAV particles in a near-native state. This capability provides highly convincing data for characterizing rAAV across different serotypes, production methods, concentrations, and purities. Cryo-EM requires minimal sample volume, with as little as 3 µL needed for a single analysis.
Precise Measurement of DNA Packaging Levels
Cryo-EM enables the direct correlation between the grayscale values of the virus particles and the DNA content inside the capsid. This allows for the precise measurement of DNA packaging levels by analyzing the differences in grayscale between the capsid and its internal contents. As a result, cryo-EM can differentiate and quantify empty, partially filled, and full capsids, providing detailed information on the relative proportions of these particles.
Accurate Identification of Functional Full Capsids
Full capsids identified through cryo-EM are not only fully packaged with a complete genome but also maintain the structural integrity necessary for gene delivery. Unlike other techniques, cryo-EM's ability to preserve the native structure of the capsids ensures that the particles are more representative of the functional, in situ capsids capable of performing therapeutic tasks.
Comprehensive Quality Attribute Characterization
Beyond empty capsid analysis, cryo-EM provides a comprehensive assessment of several critical quality attributes of rAAV particles. This includes morphology, particle size, structural integrity, and aggregation levels. These multiple data points make cryo-EM an essential tool for ensuring the overall quality and stability of rAAV preparations.
High-Resolution Structural Analysis for Advanced Research
Cryo-EM offers high-resolution structural analysis, providing three-dimensional models at the atomic level. This capability is valuable for applications such as epitope mapping and capsid engineering, enabling researchers to explore structural modifications and optimize the design of rAAV vectors for therapeutic use.
Precision, Stability, and GMP Compatibility
Cryo-EM exhibits excellent precision and stability, making it suitable for use in GMP environments. It allows for the simultaneous characterization of multiple attributes from a small sample, significantly reducing the overall analysis costs while providing reliable and reproducible results for large-scale production and quality control.
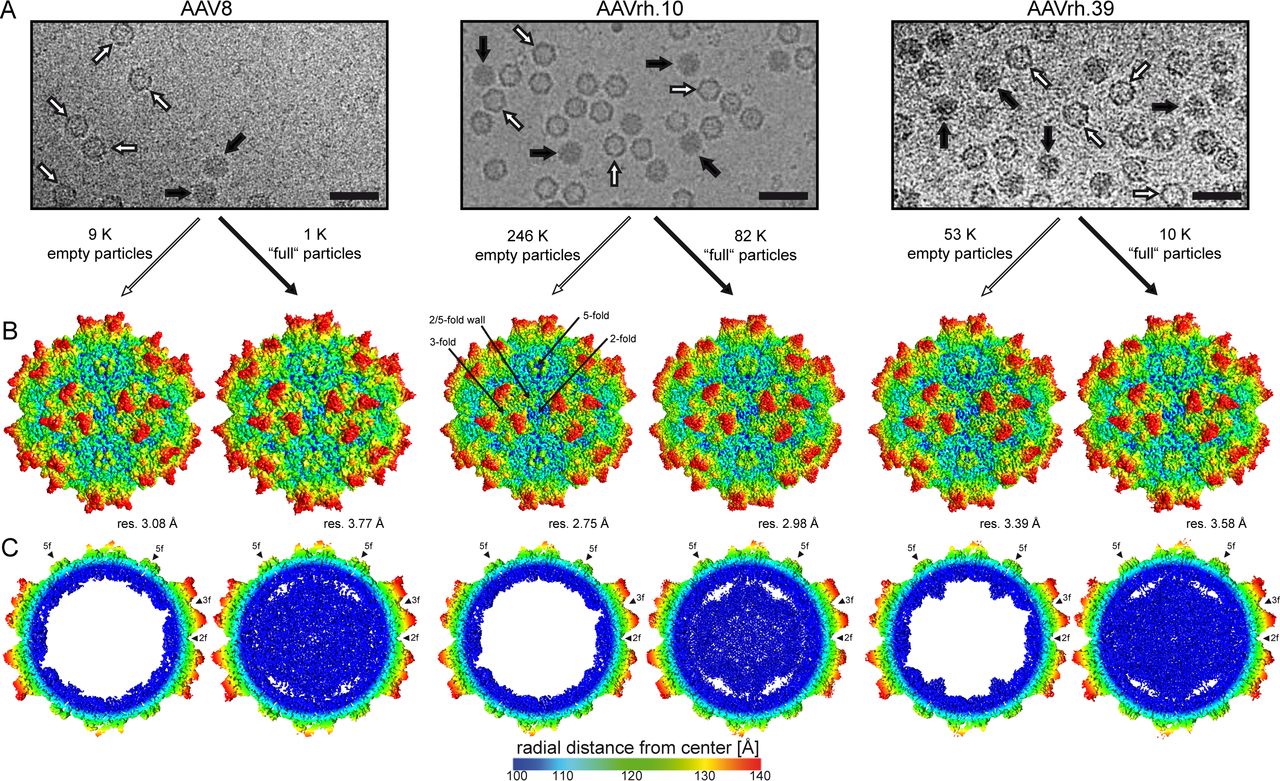 Figure 3. Structure of Empty and Genome-Containing AAV8, AAVrh.10, and AAVrh.39 Capsids. Electron micrographs showing empty (white arrow) and genome-containing (black arrow) capsids. Cryo-EM maps of the capsid surfaces and cross-sectional views are also displayed. (Mietzsch M, et al., 2020)
Figure 3. Structure of Empty and Genome-Containing AAV8, AAVrh.10, and AAVrh.39 Capsids. Electron micrographs showing empty (white arrow) and genome-containing (black arrow) capsids. Cryo-EM maps of the capsid surfaces and cross-sectional views are also displayed. (Mietzsch M, et al., 2020)
Select Service
In conclusion, the selection of the appropriate method for quantifying the empty capsid rate of rAAV should be based on the specific application requirements. Cryo-EM excels in accuracy, speed, precision, ease of use, and compliance with GMP standards. In addition to assessing the rAAV empty capsid rate, cryo-EM simultaneously evaluates other critical quality attributes, thereby reducing quality control costs.
At Creative Biostructure, our cryo-EM services support clients in all stages of rAAV research and production. We provide precise assessments of various particle forms, including empty, partially filled, and full capsids, along with aggregation profiling, structural integrity, particle size analysis, and three-dimensional structural modeling. Contact us to learn how our cryo-EM services can enhance your rAAV quality control and research efforts.
References
- Gao K, Li M, Zhong L, et al. Empty virions in AAV8 vector preparations reduce transduction efficiency and may cause total viral particle dose-limiting side effects. Molecular Therapy-Methods & Clinical Development. 2014, 1.
- Benskey M J, Sandoval I M, Manfredsson F P. Continuous collection of adeno-associated virus from producer cell medium significantly increases total viral yield. Human gene therapy methods. 2016, 27(1): 32-45.
- Li C, Samulski R J. Engineering adeno-associated virus vectors for gene therapy. Nature Reviews Genetics. 2020, 21(4): 255-272.
- Mietzsch M, Barnes C, Hull J A, et al. Comparative analysis of the capsid structures of AAVrh. 10, AAVrh. 39, and AAV8. Journal of virology. 2020, 94(6): 10.1128/jvi. 01769-19.
- Gimpel A L, Katsikis G, Sha S, et al. Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies. Molecular Therapy-Methods & Clinical Development. 2021, 20: 740-754.

-1.jpg)