Cryo-Electron Microscopy (Cryo-EM) has revolutionized structural biology, offering detailed, high-resolution insights into biomolecular structures. This powerful technique plays a critical role in drug discovery and development, aiding in the design of small molecules, antibodies, and vaccines, as well as advancing gene therapies. In this article, we'll explore how Cryo-EM is transforming research in various fields, including small molecule drug discovery, antibody development, vaccine research, and cell and gene therapies.
Cryo-EM Applications for Small Molecule Drug Discovery
Structure-Based Drug Design with Cryo-EM
Cryo-EM plays a vital role in structure-based drug design (SBDD). It can not only efficiently resolve difficult-to-study targets (such as membrane proteins and macromolecular complexes), but also provide precise structural information to guide the optimization of drug candidates.
Target Identification Using Cryo-EM
Cryo-EM technology has become an important tool for modern drug discovery because of its ability to resolve high-resolution structures of amorphous biomacromolecules. It is particularly suitable for targets that are difficult to resolve with traditional crystallography and nuclear magnetic resonance (NMR) techniques, such as membrane proteins, macromolecular complexes, and flexible protein structures.
- Membrane protein analysis: Membrane proteins are important targets in drug development, but their structural analysis has always been challenging. Cryo-EM can analyze the three-dimensional structure of membrane proteins under conditions close to physiological conditions by directly imaging frozen samples, thus providing key information for drug design.
- Analysis of macromolecular complexes: Cryo-EM can analyze the dynamic conformation and heterogeneity of protein complexes, which is difficult to achieve with traditional methods. For example, Cryo-EM has successfully analyzed the structures of a variety of protein complexes, including viruses, membrane proteins, and enzymes.
- Breakthrough in low-resolution data: Although the resolution of early Cryo-EM was low, technological advances in recent years have enabled it to reach atomic resolution (<2.5 Å) and even higher resolution (such as 1.2 Å). This allows researchers to more clearly observe ligand binding sites and their interactions with target proteins.
- Successful cases of Cryo-EM in target identification: The high-resolution structure of the viral membrane protein TRPV1 was analyzed, providing important clues for the development of new antiviral drugs.
Select Service
Related Reading
- Overview of Cryo-Electron Microscopy (Cryo-EM) Technology
- X-ray Crystallography vs Cryo-EM: Which is Best for Your Research?
- Comparison of X-ray Crystallography, NMR and EM
- Cryo-EM Applications in Protein Structure Determination
- Membrane Protein Structural Research
- MagHelix™ Structural Biology and SBDD Platform
Lead Optimization with Structural Insights
Once the high-resolution structure of the target is obtained, researchers can use this information to optimize candidate drug molecules and improve their specificity and efficacy. The precise structural data provided by Cryo-EM can help scientists understand the interaction mechanism between ligands and targets and guide subsequent drug optimization.
- Optimization of ligand binding sites: Through the structure analyzed by Cryo-EM, researchers can clarify the spatial location and conformational changes of ligand binding sites, thereby designing more effective drug molecules. For example, by observing the conformational changes of proteins after ligand binding, drug molecules can be optimized to enhance their affinity and selectivity.
- Conformational regulation of drug molecules: The activity of many proteins depends on their specific conformational states. Cryo-EM can capture these conformational changes and provide important conformational regulation strategies for drug design. For example, by regulating the conformational state of proteins, the activity of drug molecules can be enhanced or their toxicity can be inhibited.
- Multi-target drug design: Cryo-EM can also study the structures of multiple targets or complexes at the same time, providing support for multi-target drug design. For example, by analyzing the different conformational states of protein complexes, drugs that can act on multiple targets at the same time can be developed.
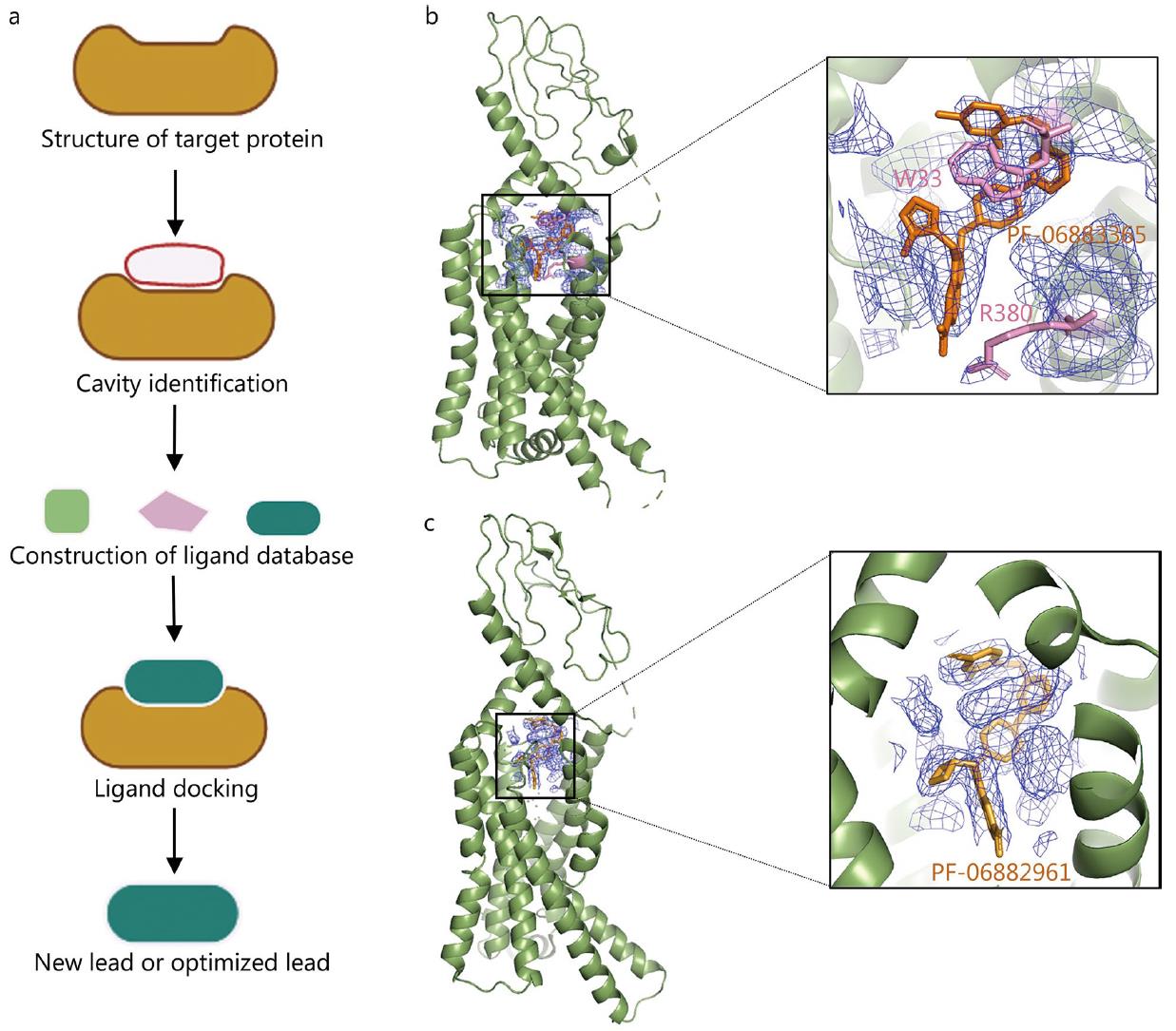 Figure 1. Application of Cryo-EM in SBDD. a. Schematic of SBDD technology. b, c. Cryo-EM structures of GLP-1R bound to small molecules PF-06883365 and PF-06882961, shown at 3.8 Å and 2.5 Å resolution, respectively, highlighting ligand binding density and key residues. (Zhu K F, et al., 2023)
Figure 1. Application of Cryo-EM in SBDD. a. Schematic of SBDD technology. b, c. Cryo-EM structures of GLP-1R bound to small molecules PF-06883365 and PF-06882961, shown at 3.8 Å and 2.5 Å resolution, respectively, highlighting ligand binding density and key residues. (Zhu K F, et al., 2023)
Fragment-Based Drug Discovery with Cryo-EM
Fragment drug discovery (FBDD) is a drug development approach that aims to identify small molecule fragments that bind to specific target proteins as the starting point for potential drug candidates. Fragment screening techniques in FBDD include methods such as X-ray crystallography, NMR spectroscopy, and surface plasmon resonance (SPR). These technologies can identify small molecule fragments that bind to target proteins and provide structural information for further optimization. However, fragment screening also faces challenges, such as the difficulty in identifying low-affinity fragments, which require further optimization to improve screening efficiency.
Fragment Screening with Cryo-EM
The low-affinity fragments obtained through fragment screening can be further optimized, for example, through strategies such as fragment growth, fragment merging and fragment assembly, to generate candidate drugs with higher affinity and specificity. Cryo-EM has become an important tool for FBDD research in recent years, especially in analyzing the binding mode of small molecules and macromolecular complexes. Cryo-EM has the potential to reveal detailed molecular interactions between fragments and proteins, and that its current reproducibility, quality, and throughput are compatible with FBDD.
Application Case 1: β-galactosidase (Bgal)
The cryo-EM structure of Bgal in complex with PETG (2.2 Å) revealed unambiguous density for the binding mode and stereochemistry of the saccharide. The structure of Bgal in complex with L-ribose (2.3 Å) showed selective binding of the pyranose form, consistent with previous X-ray structures. The binding of small molecules often results in conformational changes in the protein, which were also captured by cryo-EM.
Application Case 2: Oncology target pyruvate kinase M2 (PKM2)
Recent research performed a fragment screen of 68 fragments on PKM2, demonstrating that small nonplanar molecules could be visualised using cryo-EM. The PKM2/L-threonine co-complex (2.6 Å) and PKM2/Cmp5 (3.2 Å) co-complexes highlighted the challenges of fragment size and structure resolution. The study also evaluated the behavior of fragment cocktails in cryo-EM samples, showing that cocktails of four ligands could be screened without issues.
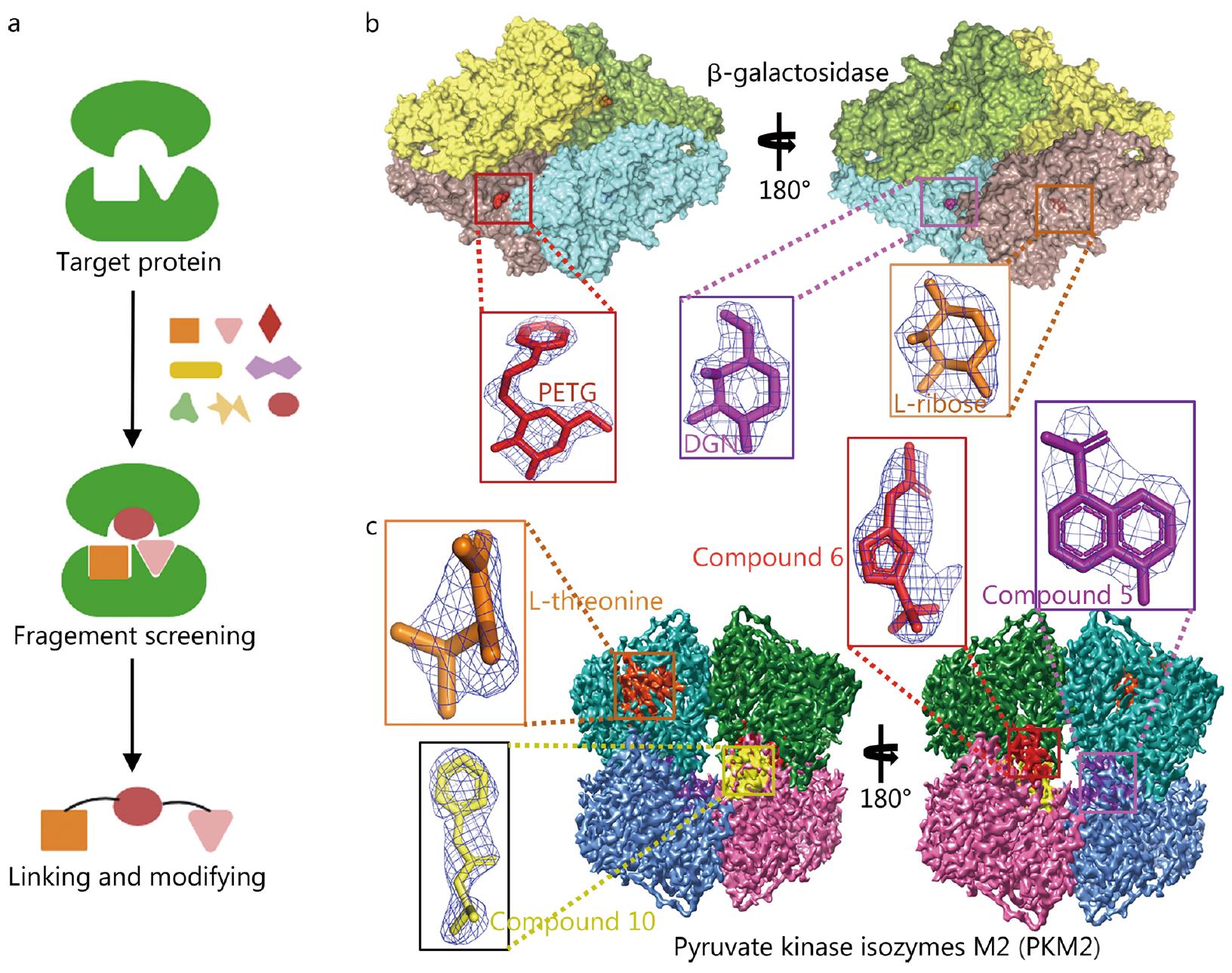 Figure 2. Application of Cryo-EM in FBDD. (a) Schematic diagram of FBDD technology. (b) Cryo-EM 3D map of Bgal, with active sites highlighted and ligands PETG, L-ribose, and DGN fitted to their respective densities. (c) Cryo-EM 3D map of PKM2, with compound binding sites and ligands L-threonine, compound 5, 6, and 10 fitted to their densities. (Zhu K F, et al., 2023)
Figure 2. Application of Cryo-EM in FBDD. (a) Schematic diagram of FBDD technology. (b) Cryo-EM 3D map of Bgal, with active sites highlighted and ligands PETG, L-ribose, and DGN fitted to their respective densities. (c) Cryo-EM 3D map of PKM2, with compound binding sites and ligands L-threonine, compound 5, 6, and 10 fitted to their densities. (Zhu K F, et al., 2023)
Importance of Structural Insights for FBDD
The high-resolution structural information obtained through Cryo-EM can provide the following important support for FBDD:
- Clear binding sites: Cryo-EM can reveal the precise binding mode between fragments and target proteins, helping researchers to identify key binding sites.
- Optimize drug design: Combined with Cryo-EM structural information, it can guide subsequent chemical modification and fragment optimization work to improve the affinity and specificity of candidate drugs.
- Explore new targets: Cryo-EM can also be used to analyze the structure of target proteins that have not been fully studied, opening up new research directions for FBDD.
Targeted Protein Degradation with Cryo-EM
Targeted protein degradation is an emerging drug discovery and treatment strategy that removes specific disease-related proteins by utilizing intracellular protein degradation mechanisms such as the ubiquitin-proteasome system and the lysosomal pathway. This technology overcomes the limitations of traditional small molecule drugs in target selectivity, drug resistance, and bioavailability, and provides a new therapeutic approach for "undruggable" targets.
Main Strategies for Targeted Protein Degradation
- PROTAC (Proteolysis-Targeting Chimeras): PROTAC molecules are bifunctional small molecules that induce the target protein to be marked and degraded by binding to the target protein and E3 ubiquitin ligase at the same time.
- Molecular Glues: Promote the degradation of the target protein by changing the conformation of the protein or increasing its affinity with the E3 ligase.
- Other strategies: including autophagy-targeted chimeras (AUTAC), lysosome-targeted chimeras (LYTAC), etc.
Select Service
Cryo-EM in PROTAC Research
The application of cryo-EM technology in PROTAC research is mainly reflected in the following aspects:
- Structural analysis: Cryo-EM can analyze the complex structure of PROTAC molecules, target proteins and E3 ligases, providing high-resolution three-dimensional images for understanding their mechanism of action.
- Optimized design: By analyzing the structure of PROTAC binding to the target protein, researchers can optimize the design of PROTAC molecules and improve their selectivity and efficacy.
- New target discovery: Cryo-EM can also be used to screen targets that have not been fully studied, opening up new directions for drug discovery.
Specific cases:
- BacPROTAC: The structure of the BacPROTAC and ClpCP protein complex was analyzed using cryo-EM, revealing its mechanism of action in bacteria.
- Antibody-mediated PROTAC (AbTAC): The binding mode of AbTAC and RNF43 was analyzed by cryo-EM, further verifying its potential for application in immunotherapy.
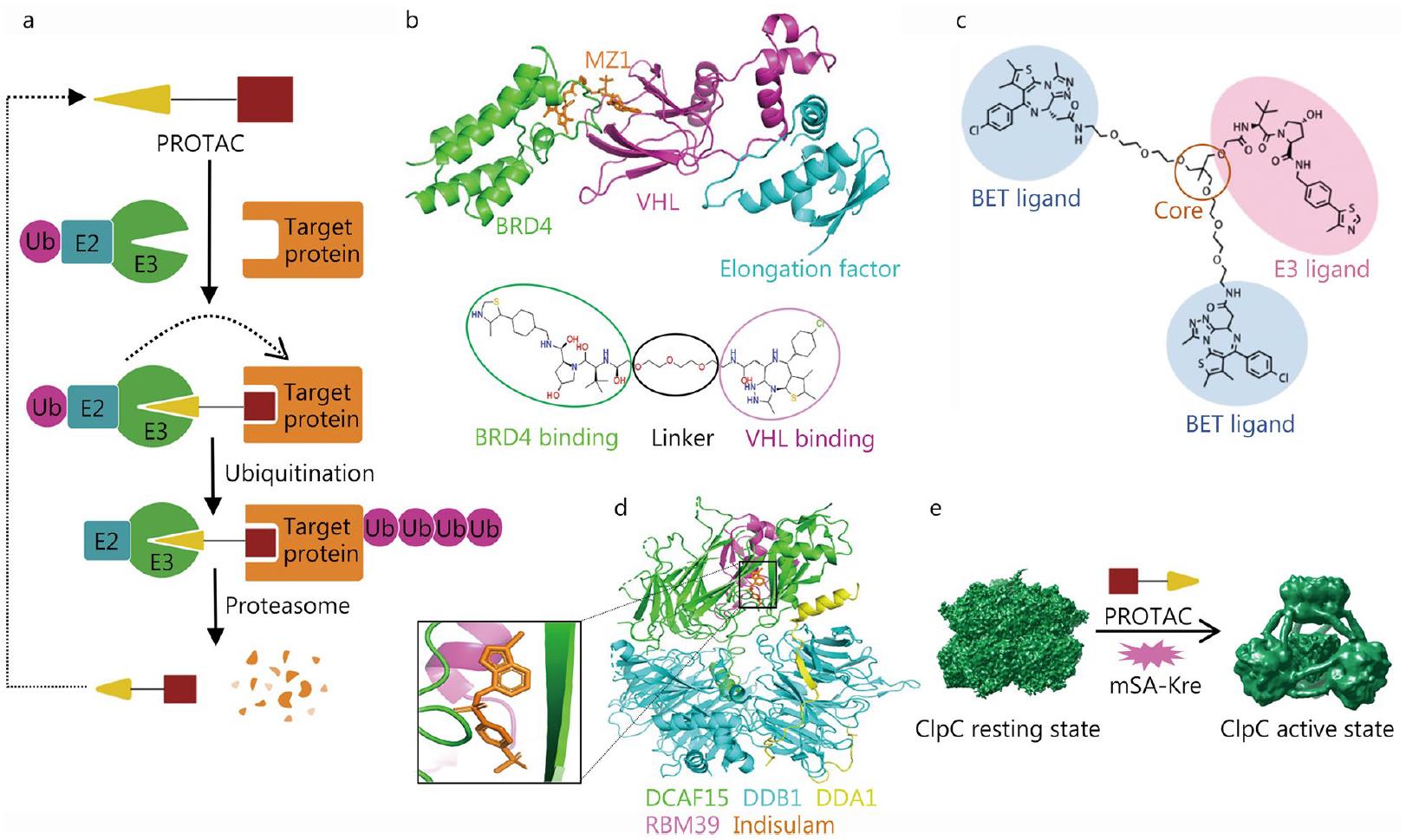 Figure 3. Application of Cryo-EM in PROTAC. (a) Schematic diagram of PROTAC technology. (b) Cartoon of the MZ1-mediated BRD4-VHL complex and MZ1 design. (c) Structure of the trivalent PROTAC. (d) Cartoon of the human DCAF15-DDB1-DDA1-RBM39 complex with indisulam. (e) BacPROTAC-mediated ClpC activation for protein degradation. (Zhu K F, et al., 2023)
Figure 3. Application of Cryo-EM in PROTAC. (a) Schematic diagram of PROTAC technology. (b) Cartoon of the MZ1-mediated BRD4-VHL complex and MZ1 design. (c) Structure of the trivalent PROTAC. (d) Cartoon of the human DCAF15-DDB1-DDA1-RBM39 complex with indisulam. (e) BacPROTAC-mediated ClpC activation for protein degradation. (Zhu K F, et al., 2023)
Cryo-EM and GPCR Drug Discovery
High-Resolution Structures of GPCRs with Cryo-EM
Cryo-EM technology has made significant progress in GPCR structural analysis, especially in the high-resolution structural analysis of important GPCRs such as β2-adrenergic receptor and GLP-1 receptor. For example:
- β2-adrenergic receptor: Through Cryo-EM technology, researchers have analyzed the complex structure of β2-adrenergic receptor and different G proteins (such as Gs protein), revealing its activation mechanism and ligand binding mode.
- GLP-1 receptor: Cryo-EM technology has successfully analyzed the complex structure of GLP-1 receptor with small molecule agonists, antagonists and positive regulators, providing important information for understanding its ligand recognition and signal transduction mechanism.
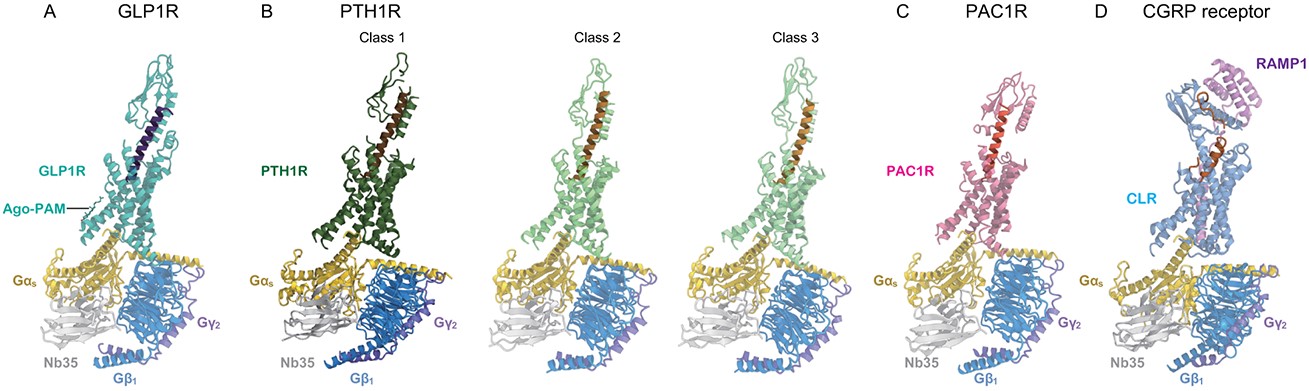 Figure 4. Cryo-EM structures of Class B GPCR-G-Protein Complexes. (A) GLP1R structure bound to GLP1 and ago-PAM. (B) Three PTH1R classes with different ECD orientations. (C) PAC1R-PACAP38 complex. (D) CGRP receptor-CGRP complex. (Shihoya W, et al., 2024)
Figure 4. Cryo-EM structures of Class B GPCR-G-Protein Complexes. (A) GLP1R structure bound to GLP1 and ago-PAM. (B) Three PTH1R classes with different ECD orientations. (C) PAC1R-PACAP38 complex. (D) CGRP receptor-CGRP complex. (Shihoya W, et al., 2024)
Dynamic Conformation and Allosteric Regulation
Cryo-EM technology can capture the dynamic conformation of GPCR in different states, which is crucial for understanding the allosteric regulation and signal transduction of GPCR:
- CGRP receptor: Cryo-EM was used to analyze the structure of CGRP receptor in different states, revealing its binding mode with small molecule inhibitors and showing its dynamic changes in signal transduction.
- δ-opioid receptor: The study discovered the structure of the δ-opioid receptor complex with Gi protein, further revealing its mechanism of action in drug regulation.
Select Service
Related Reading
- Structural Research of G Protein-coupled Receptors (GPCRs) Class A
- Structural Research of G Protein-coupled Receptors (GPCRs) Class B1
- Structural Research of G Protein-coupled Receptors (GPCRs) Class B2
- Structural Research of G Protein-coupled Receptors (GPCRs) Class C
- Structural Research of G Protein-coupled Receptors (GPCRs) Class D
- Structural Research of G Protein-coupled Receptors (GPCRs) Class F
Cryo-EM Applications in Antibody Discovery
The application of Cryo-EM technology in antibody discovery and therapeutic antibody development, especially in high-resolution epitope mapping and structural insights, has significant advantages and potential.
What Is Antibody Discovery
Antibody discovery refers to the process of identifying and optimizing antibodies that can specifically bind to target antigens through a series of technical means. This process usually includes steps such as target evaluation, antibody screening, functional optimization and engineering, and ultimately generates antibodies with high affinity, specificity and stability.
Antibody Discovery Workflow
Antibody discovery usually includes the following key steps:
- Target assessment: Identify the antigen or disease-related pathway that needs to be targeted.
- Antibody generation: Generate a large number of antibody libraries through technical means (such as hybridoma technology, phage display technology, etc.).
- Screening and optimization: Use high-throughput screening technology to screen candidate antibodies with high affinity, specificity and functionality from millions or even billions of antibodies.
- Antibody engineering: Affinity maturation, humanization, Fc engineering and other optimizations are performed on the screened antibodies to improve their stability and functionality.
- Clinical development: Apply the optimized antibodies to preclinical and clinical trials, and finally achieve drugization.
Leveraging Cryo-EM for Antibody Discovery
Cryo-EM technology has become a key tool to accelerate the development of antibody drugs, and its application runs through the entire process, from target screening to the development of therapeutic antibodies. It can quickly and efficiently provide high-resolution structural information of antibody-antigen interactions, thereby helping to understand the mechanism of action and guide subsequent antibody optimization. In addition, the advantages of cryo-EM technology in antibody discovery include:
- Rapid generation of structural information: Through single particle analysis (SPA) technology, cryo-EM can resolve the three-dimensional structure of the antibody-antigen complex in a short time.
- Support multivalent antibody design: The structural information provided by cryo-EM helps to design bispecific antibodies and ADCs (antibody drug conjugates), which can significantly improve the therapeutic effect.
- Improve the efficiency of vaccine development: By resolving the high-resolution structure of antigen epitopes bound to antibodies, cryo-EM can optimize vaccine design and enhance immunogenicity.
Select Service
Related Reading
High-Resolution Epitope Mapping
Epitope mapping is an important step in antibody research to determine how antibodies recognize antigens. Cryo-EM technology shows unique advantages in this field:
- High-resolution epitope localization: Cryo-EM can maintain native conformation under near-physiological conditions, thereby achieving accurate mapping of epitope-antigen interactions.
- Revealing dynamic changes: Cryo-EM can capture the dynamic behavior of antibody-antigen complexes, such as conformational changes of antibody molecules after antigen binding.
- Supporting multivalent antibody development: By analyzing the three-dimensional structures of different epitopes, cryo-EM helps develop multivalent antibodies that can simultaneously target multiple antigen epitopes.
Structural Insights for Therapeutic Antibodies
The high-resolution structural information provided by cryo-EM is crucial for the development of therapeutic antibodies:
- Revealing the mechanism of action: By analyzing the detailed structure of antibody-antigen binding, cryo-EM can reveal how antibodies neutralize pathogens or inhibit the function of disease-related proteins.
- Optimizing antibody design: Cryo-EM data can be used to guide antibody engineering, such as by adjusting the epitope specificity of antibodies to improve their affinity and selectivity.
- Supporting drug repurposing: Cryo-EM can also be used to explore how existing drug molecules interact with target proteins, thereby providing new ideas for drug repurposing.
In addition, cryo-EM technology can also be combined with mass spectrometry technology for antibody sequencing and epitope mapping, further improving the efficiency and accuracy of antibody discovery. For example, single-particle cryo-EM and mass spectrometry-based substrate-dependent proteomics technology can simultaneously perform antibody sequencing and epitope mapping, thereby more comprehensively characterizing the antibody library.
Select Service
Case Studies of Cryo-EM in Therapeutic Antibody Development
1. Development of anti-HIV antibodies: Using polyclonal antibodies induced by BG505 SOSIP.T33-31 nanoparticle immunogens, antibodies with V1/V2/V3 domains were studied by cryo-EM/in situ ligand binding analysis.
2. Development of anti-influenza virus antibodies: The report introduces the cryo-EM structural analysis of a new antibody drug, which is formed by the combination of 16.ND.92 Fab fragment and SI/06 HA.
3. Development of anti-PDK1 antibodies: The complexes of different ligands with PDK1 were studied, and the application of cryo-EM in screening fragments was demonstrated.
4. Development of anti-tumor antibodies: The structures of various anti-tumor antibodies were analyzed using cryo-EM technology, providing important structural information for drug design.
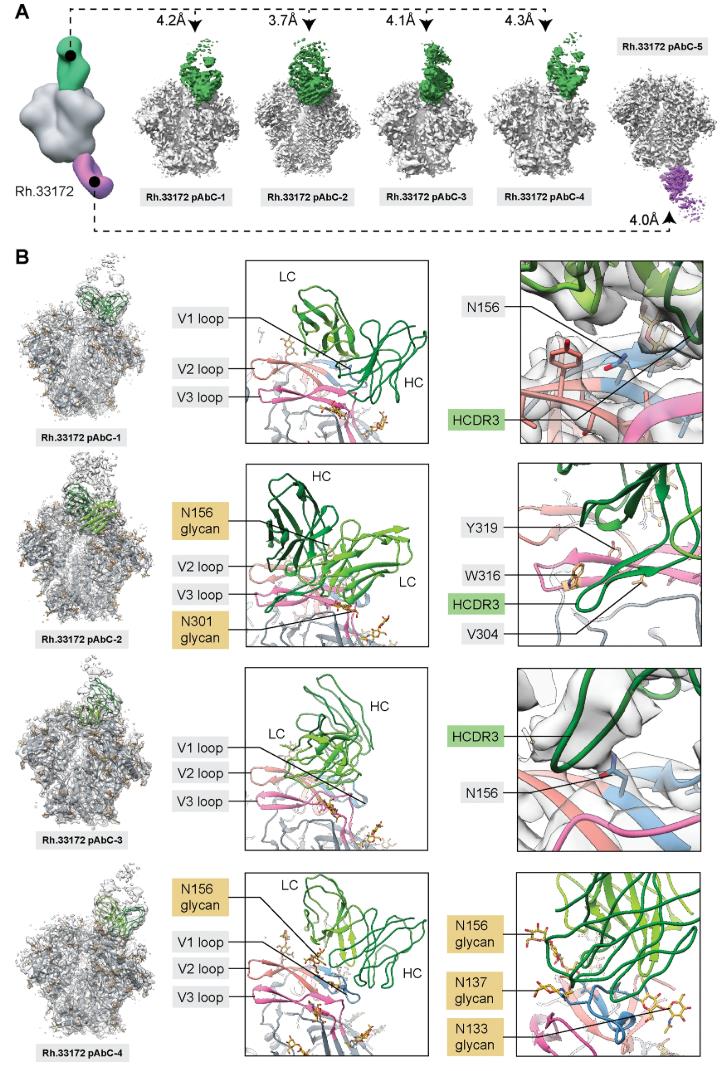 Figure 5. Cryo-EM Analysis of Polyclonal Antibodies Elicited by BG505 SOSIP-T33-31 Nanoparticle Immunogen. (A) Cryo-EM analysis of immune complexes formed with polyclonal Fabs from animal Rh.33172, showing BG505 SOSIP antigen in gray. (B) Structural models of four V1/V2/V3-targeting pAbs, with the ribbon representation on the left and close-ups highlighting epitope/paratope components. V1 loop (blue), V2 loop (salmon), and V3 loop (magenta) are indicated, with inferred heavy (dark green) and light (light green) chains. (Antanasijevic A, et al., 2021)
Figure 5. Cryo-EM Analysis of Polyclonal Antibodies Elicited by BG505 SOSIP-T33-31 Nanoparticle Immunogen. (A) Cryo-EM analysis of immune complexes formed with polyclonal Fabs from animal Rh.33172, showing BG505 SOSIP antigen in gray. (B) Structural models of four V1/V2/V3-targeting pAbs, with the ribbon representation on the left and close-ups highlighting epitope/paratope components. V1 loop (blue), V2 loop (salmon), and V3 loop (magenta) are indicated, with inferred heavy (dark green) and light (light green) chains. (Antanasijevic A, et al., 2021)
Cryo-EM Applications in Vaccine Research
Cryo-EM has become a pivotal tool in vaccine research, offering high-resolution structural insights that are essential for the design, optimization, and development of vaccines. The role of Cryo-EM in vaccine development is mainly reflected in the following aspects:
High-Resolution Structural Analysis and Antigen Design
Cryo-EM can analyze the structure of biological macromolecules, including viruses, antibody-antigen complexes, etc., at near-atomic resolution. This high-resolution structural information is crucial for understanding the molecular mechanism of pathogens and also provides a solid foundation for structure-based vaccine design. Through Cryo-EM, the three-dimensional structure of pathogen surface proteins, including their conformation and dynamic changes, can be understood in detail. For example, in HIV vaccine design, researchers used Cryo-EM to analyze the structure of HIV-1 envelope protein, and through modeling and optimization, designed vaccine components that can effectively induce immune responses. In addition, Cryo-EM can also be used to analyze the structure of other pathogens, such as the pre-fusion conformation of RSV and HIV, providing key information for vaccine design. By analyzing the binding pattern of HCMV (cytomegalovirus) gB protein with antibodies, researchers were able to optimize antigen design and improve vaccine effectiveness.
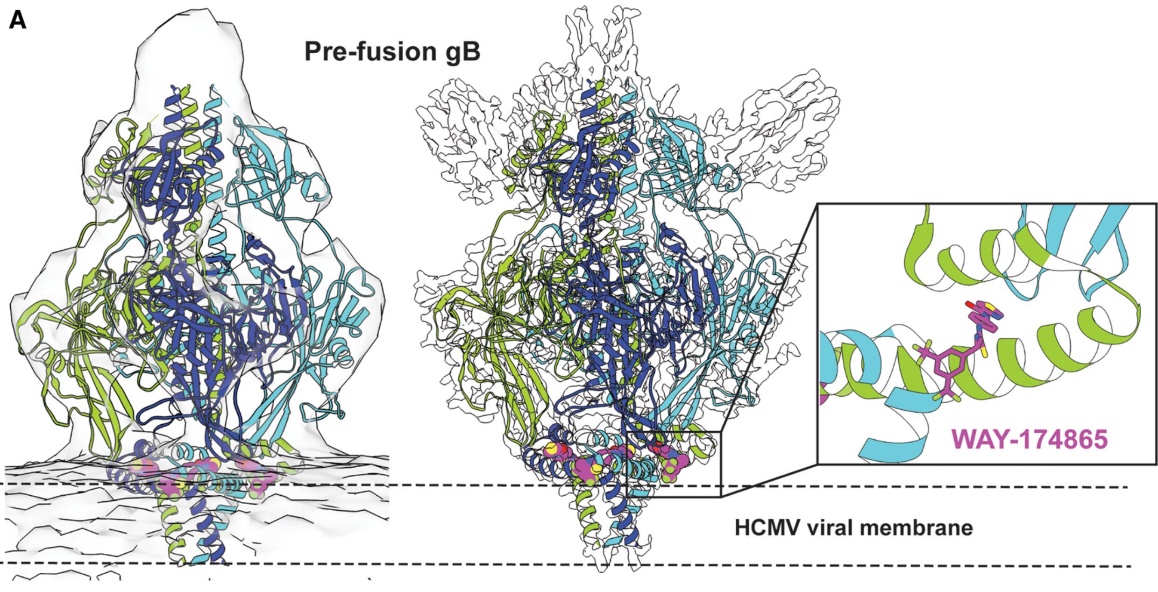 Figure 6. Cryo-EM Structure of Pre-Fusion-Stabilized HCMV gB Trimer Bound to WAY-174865. Cryo-EM structure of HCMV gB trimer (cartoon) bound to WAY-174865 (PDB 7KDP), shown with tomographic reconstruction (left), single-particle cryo-EM map (middle), and binding pocket of WAY-174865 (right inset). Unmodeled densities correspond to a neutralizing antibody Fab (SM5-1). (Lees J A, et al., 2021)
Figure 6. Cryo-EM Structure of Pre-Fusion-Stabilized HCMV gB Trimer Bound to WAY-174865. Cryo-EM structure of HCMV gB trimer (cartoon) bound to WAY-174865 (PDB 7KDP), shown with tomographic reconstruction (left), single-particle cryo-EM map (middle), and binding pocket of WAY-174865 (right inset). Unmodeled densities correspond to a neutralizing antibody Fab (SM5-1). (Lees J A, et al., 2021)
Immunogenicity Studies
Cryo-EM can also be used to study the immunogenicity of vaccines, by analyzing the binding sites of neutralizing and non-neutralizing antibodies, revealing the potential immunogenicity of antigens. For example, in the study of HPV vaccines, Cryo-EM revealed the density and glycosylation sites of the L2 protein, as well as the mechanism by which different antibodies interact with it. This information helps to optimize vaccine design and improve its immune effect.
Accelerate Vaccine Development Process
Cryo-EM technology has greatly shortened the time from discovery to application in vaccine development. Traditional X-ray crystallography requires a large amount of purified homogenous samples, while Cryo-EM does not require a crystallization process, so structural information can be obtained more quickly. For example, during the COVID-19 epidemic, Cryo-EM helped scientists quickly resolve the structure of the SARS-CoV-2 spike protein and designed a variety of vaccines based on this information.
Support Development of Multiple Vaccine Forms
Cryo-EM is not only suitable for traditional inactivated vaccines and live attenuated vaccines, but also supports the development of new vaccine forms, such as mRNA vaccines and viral vector vaccines. By analyzing the structure of key components of these vaccines (such as mRNA, viral particles, etc.), Cryo-EM provides important support for optimizing vaccine design.
Support Lipid Nanoparticle (LNP) Characterization
In the process of vaccine manufacturing and formulation, lipid nanoparticles (LNPs) are one of the key carriers. As non-viral carriers, LNPs can deliver vaccine components to specific cells or tissues, thereby improving the safety and effectiveness of vaccines. Cryo-EM can intuitively visualize the structure of LNPs, including its shape, size, load, integrity and other properties. This information is crucial for optimizing vaccine formulations and improving vaccine effectiveness.
Customization of Personalized Vaccines
Through Cryo-EM technology, high-precision analysis of pathogen structures of different individuals can be achieved, thus providing a basis for personalized vaccine design. For example, in the development of COVID-19 vaccines, Cryo-EM analyzed the structures of SARS-CoV-2 viral antigens BNT162b1 and BNT162b2, and further optimized the design of the vaccine by combining imaging technology. The application of this technology is not limited to a single pathogen, but can also be extended to multiple pathogens to achieve more extensive personalized vaccine customization.
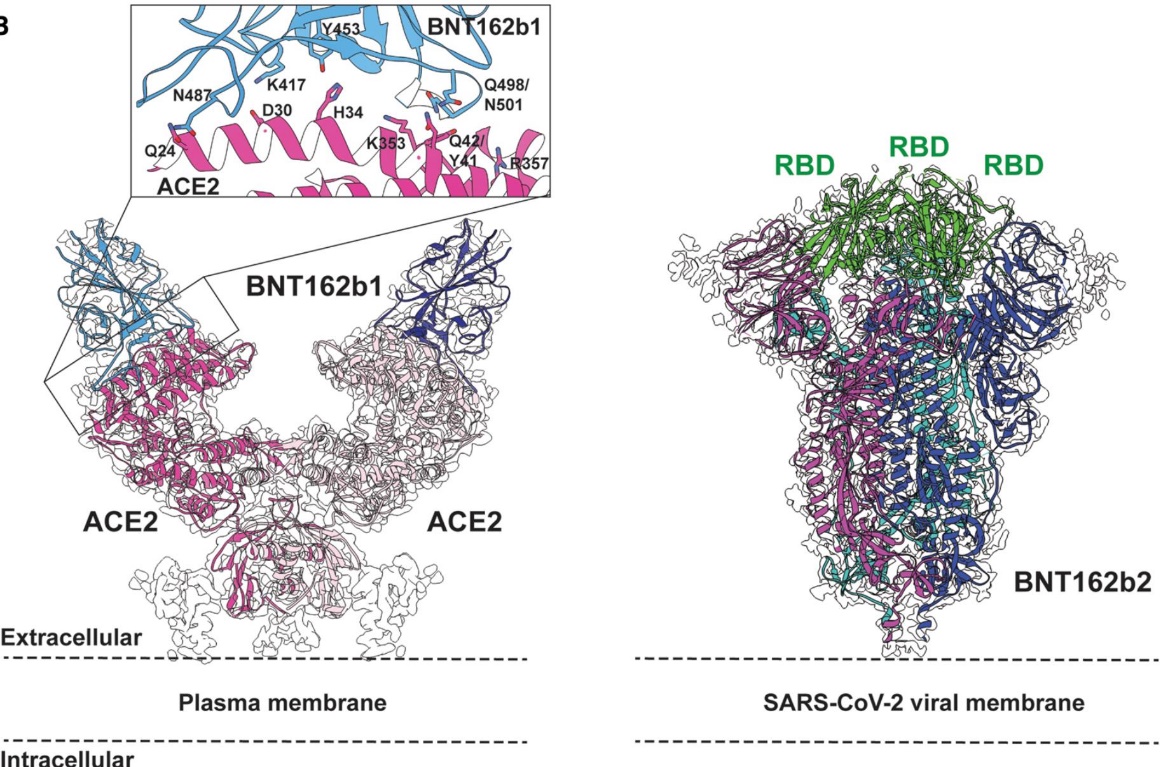 Figure 7. Cryo-EM Structures of SARS-CoV-2 Vaccine Antigens BNT162b1 and BNT162b2. Left: Cryo-EM structure of dimeric ACE2 (pink) bound to BNT162b1-encoded SARS-CoV-2 spike RBD (blue, PDB 7L7F), with its cryo-EM map (EMD-23211) and ACE2-RBD binding interface. Right: Cryo-EM structure of BNT162b2-encoded pre-fusion-stabilized SARS-CoV-2 spike (PDB 7L7K), with cryo-EM density (EMD-23215), subunits colored individually and RBD in green. (Lees J A, et al., 2021)
Figure 7. Cryo-EM Structures of SARS-CoV-2 Vaccine Antigens BNT162b1 and BNT162b2. Left: Cryo-EM structure of dimeric ACE2 (pink) bound to BNT162b1-encoded SARS-CoV-2 spike RBD (blue, PDB 7L7F), with its cryo-EM map (EMD-23211) and ACE2-RBD binding interface. Right: Cryo-EM structure of BNT162b2-encoded pre-fusion-stabilized SARS-CoV-2 spike (PDB 7L7K), with cryo-EM density (EMD-23215), subunits colored individually and RBD in green. (Lees J A, et al., 2021)
Cryo-EM Applications in Cell and Gene Therapies
Cryo-EM not only provides high-resolution protein structure information, but also supports the rational design and optimization of CAR proteins and gene delivery vectors. These technological advances provide a solid scientific basis for improving the safety and efficacy of CAR T cell therapy and gene therapy.
Application of Cryo-EM in CAR T Cell Therapy
CAR T cell therapy is an immunotherapy that uses the patient's own T cells to recognize and eliminate cancer cells. Its core is the chimeric antigen receptor (CAR), a modular receptor composed of immune elements that can accurately recognize specific antigens and activate downstream signaling pathways to kill cancer cells. However, the success of CAR T cell therapy depends on its high affinity and specific antigen binding ability, which requires in-depth research on the three-dimensional structure of the CAR protein. The application of cryo-EM technology in CAR T cell therapy is mainly reflected in the following aspects:
- High-Resolution Structural Analysis of CAR Protein: Cryo-EM can analyze the three-dimensional structure of complex protein complexes at near-atomic resolution, which is crucial for understanding the interaction between CAR protein and target antigen. For example, by analyzing the structure of the CAR protein and CD19 complex by cryo-EM, researchers can reveal its intermolecular interactions and conformational changes, thereby providing a theoretical basis for designing CAR proteins with higher affinity and specificity.
- Optimizing CAR Protein Design: Cryo-EM not only helps to analyze the structure of CAR proteins, but also supports the optimization of their performance through structure-guided methods. For example, by studying the structural differences of different CAR proteins through cryo-EM, researchers can find the key amino acid residues that affect their functions and improve the design of CAR proteins accordingly to improve their efficiency and safety in clinical applications.
- Solving the Stability Problem of CAR Proteins: CAR proteins may face the problem of insufficient stability in practical applications, and cryo-EM can help researchers identify the key factors that affect protein stability and improve their stability through structural optimization.
- Support Non-Viral Vector Development: In addition to traditional viral vectors, cryo-EM has also been used to study the structural properties of non-viral vectors (such as lipid nanoparticles). These vectors can more effectively deliver CAR genes into T cells while reducing immunogenicity and toxicity issues.
Characterization of Gene Delivery Vectors Using Cryo-EM
Gene therapy relies on the precise delivery of therapeutic genes into target cells. The applications of cryo-EM in this field mainly include the following aspects:
- High-Resolution Structural Analysis of Viral Vectors: Viral vectors (such as adeno-associated virus AAV, lentivirus, etc.) are one of the most commonly used tools in gene therapy. Cryo-EM can analyze the high-resolution structure of viral vectors, including their surface decoration, the conformation of envelope proteins, and the binding mode with target cell receptors. This information helps to optimize the design of viral vectors and improve their targeting and safety.
- Structural Characterization of Non-Viral Vectors: Non-viral vectors (such as lipid nanoparticles) have attracted attention due to their non-immunogenicity and low toxicity. Cryo-EM can analyze the dynamic structural properties of these vectors, help researchers understand how they interact with target cells, and guide their further optimization.
- Improvement of Gene Editing Tools: Gene editing tools such as CRISPR-Cas9 play an important role in gene therapy. Cryo-EM can analyze the molecular structure of these tools to optimize their performance. For example, by studying the binding mechanism of Cas9 protein and sgRNA through cryo-EM, researchers can design more efficient gene editing systems.
- Reduce Off-Target Effects: Cryo-EM can also help researchers understand the off-target effects that may occur during gene editing and reduce these risks through optimized design.
Select Service
In conclusion, Cryo-EM is an invaluable tool in modern drug discovery and therapeutic development. Its ability to provide high-resolution structures of biomolecules accelerates the design of effective drugs, antibodies, vaccines, and gene therapies. At Creative Biostructure, we offer comprehensive cryo-EM services for drug discovery tailored to your research needs. Contact us to learn how we can support your drug development and therapeutic innovations.
References
- Li N, Li Z, Fu Y, et al. Cryo-EM studies of virus-antibody immune complexes. Virologica Sinica. 2020, 35(1): 1-13.
- Saur M, Hartshorn M J, Dong J, et al. Fragment-based drug discovery using cryo-EM. Drug Discovery Today. 2020, 25(3): 485-490.
- Antanasijevic A, Sewall L M, Cottrell C A, et al. Polyclonal antibody responses to HIV Env immunogens resolved using cryoEM. Nature Communications. 2021, 12(1): 4817.
- Lees J A, Dias J M, Han S. Applications of Cryo-EM in small molecule and biologics drug design. Biochemical Society Transactions. 2021, 49(6): 2627-2638.
- Zhu K F, Yuan C, Du Y M, et al. Applications and prospects of cryo-EM in drug discovery. Military Medical Research. 2023, 10(1): 10.
- Ouyang W O, Lv H, Liu W, et al. Rapid synthesis and screening of natively paired antibodies against influenza hemagglutinin stem via oPool+ display. bioRxiv, 2024.
- Shihoya W, Iwama A, Sano F K, et al. Cryo-EM advances in GPCR structure determination. The Journal of Biochemistry. 2024, 176(1): 1-10.

-1.jpg)