Introduction: Cryo EM vs TEM at a Glance
Electron microscopy has revolutionized our understanding of biological structures, cellular organization, and material properties at the nanoscale level. While Transmission Electron Microscopy (TEM) represents a broader family of electron imaging techniques, Cryo-Electron Microscopy (Cryo-EM) emerges as a specialized approach that has transformed structural analysis across multiple scientific disciplines. Understanding their distinct capabilities and applications is crucial for researchers in structural biology, cell biology, and materials science to make informed decisions for their specific research needs.
Key Highlights:
- TEM offers versatile applications across biological and material sciences
- Cryo-EM specializes in preserving and analyzing samples in their native state
- Both techniques complement each other in modern research settings
- Sample preparation methods dictate the type of information that can be obtained.
| Features | Cryo-EM | Conventional TEM |
| Sample Type |
|
|
| Sample Preparation | Flash-freezing of samples in vitreous ice, minimal preparation | Chemical fixation, staining, or polymer embedding |
| Research Objectives |
|
|
| Sample State | Native state preserved in vitreous ice | Fixed state with enhanced contrast |
| Resolution Needs |
|
|
| Technological Requirements | Advanced direct electron detectors, automated systems | Standard electron detectors, various imaging modes |
| Key Advantages |
|
|
The Evolution from TEM to Cryo-EM
The journey of electron microscopy in scientific research represents a remarkable evolution in our ability to visualize and understand structures at the nanoscale level.
TEM as the Foundational Technology
TEM emerged as a groundbreaking advancement in microscopy science, marking a significant leap from traditional light microscopy. Initially developed to overcome the resolution limitations of light microscopes, TEM introduced several revolutionary capabilities:
- Early development focused on materials science applications, with pioneering work in metal and crystal structure analysis
- Introduction of chemical fixation and staining techniques enabled detailed visualization of biological specimens
- Progressive improvements in electron sources and lens systems enhanced image resolution and contrast
- Integration of digital imaging systems revolutionized data collection and analysis capabilities
Development of Cryo-EM as a Specialized Technique
The emergence of Cryo-EM represented a paradigm shift in structural biology, addressing the fundamental challenges of studying biological samples in their native state:
- Recognition of sample preservation issues in conventional TEM led to exploration of alternative preparation methods
- Development of vitrification techniques allowed samples to be frozen without destructive ice crystal formation
- Introduction of automated data collection systems enabled analysis of multiple particle orientations
- Advanced image processing algorithms made it possible to reconstruct 3D structures from 2D projections
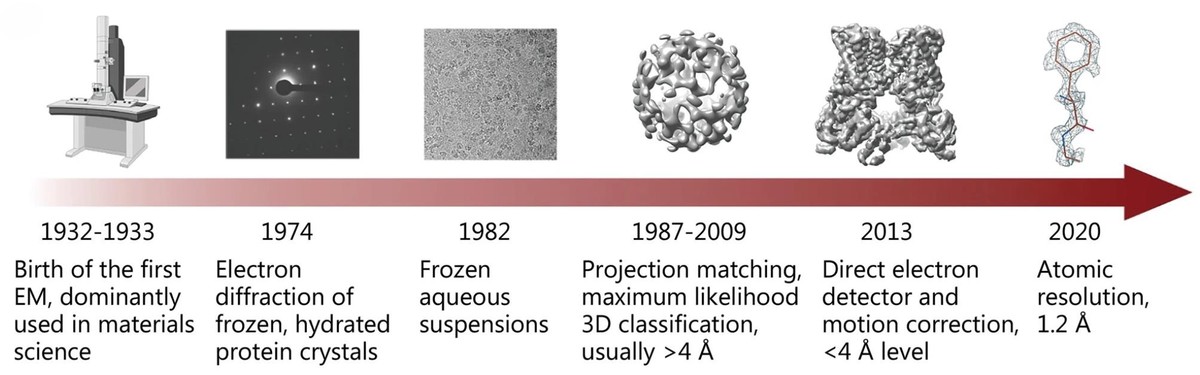 Figure 1. History of single-particle cryo-EM development. (Zhu K F, et al., 2023)
Figure 1. History of single-particle cryo-EM development. (Zhu K F, et al., 2023)
Core Differences in TEM and Cryo-EM
Sample Preparation Methods
Understanding the fundamental differences in sample preparation between TEM and Cryo-EM is crucial for researchers to obtain optimal results in their studies.
Conventional TEM Sample Preparation
- Chemical Fixation Process: The foundation of TEM sample preparation lies in chemical fixation, where specimens undergo a series of chemical treatments. This process typically begins with cross-linking agents like glutaraldehyde to preserve cellular structures, followed by osmium tetroxide treatment for membrane stabilization. The samples then go through a careful dehydration process using ethanol gradients, resulting in well-preserved structures though chemically modified from their native state.
- Staining Techniques: Staining in TEM serves the critical purpose of enhancing contrast for better visualization. The process employs various heavy metal salts, with negative staining being particularly popular for macromolecular studies. While this approach significantly improves image contrast, researchers must be mindful of potential artifacts introduced during the staining process.
- Sample Processing and Storage: The final stages of TEM sample preparation involve embedding specimens in resin and creating ultrathin sections. This process allows for long-term sample storage and provides the structural support necessary for detailed examination. The ability to process samples at room temperature simplifies handling procedures but may affect certain delicate molecular structures.
Select Service
Related Reading
Cryo-EM Sample Preparation
- Vitrification Process: At the heart of Cryo-EM sample preparation is the vitrification process, where specimens are rapidly frozen in liquid ethane at approximately -188°C. This crucial step creates vitreous ice without crystallization, effectively preserving the sample's native structure with minimal chemical modification. The speed and precision of this process are critical for successful sample preparation.
- Grid Preparation and Optimization: The preparation of EM grids for Cryo-EM requires meticulous attention to detail. Researchers must carefully control sample thickness and ice formation to achieve optimal imaging conditions. This process involves precise application of samples to specialized grids and careful monitoring of environmental conditions during preparation.
- Sample Handling and Transfer: Maintaining sample integrity in Cryo-EM requires rigorous attention to the cold chain. Specimens must be kept at cryogenic temperatures throughout handling and transfer processes, utilizing specialized equipment and procedures to prevent ice contamination and maintain sample quality.
Select Service
Impact on Sample Integrity and Research Outcomes
- Structural Preservation Analysis
The choice between TEM and Cryo-EM significantly impacts structural preservation. While TEM excels in providing detailed cellular ultrastructure, Cryo-EM maintains native protein conformations and molecular interactions, offering unique insights into biological systems in their natural state.
- Resolution and Data Quality
Each method presents distinct advantages in terms of resolution and data quality. TEM provides robust contrast for cellular components, making it ideal for ultrastructural studies. Cryo-EM, while more challenging in terms of contrast, enables the visualization of molecular details at near-atomic resolution, particularly valuable for structural biology applications.
- Application-Specific Considerations
The selection of preparation method should be guided by specific research requirements. TEM preparation techniques are well-suited for a broad range of samples, including tissues and materials science specimens. Cryo-EM preparation is particularly advantageous for studying purified proteins and macromolecular complexes in their native states.
TEM and Cryo-EM Imaging Mechanisms
The selection of preparation method should be guided by specific research requirements. TEM preparation techniques are well-suited for a broad range of samples, including tissues and materials science specimens. Cryo-EM preparation is particularly advantageous for studying purified proteins and macromolecular complexes in their native states.
Shared Fundamental Principles
- Electron Beam Generation and Control
Both TEM and Cryo-EM rely on the fundamental principle of electron beam interaction with specimens. The process begins with an electron source, typically a field emission gun, generating a coherent beam of electrons. These electrons are then precisely controlled through a series of electromagnetic lenses that focus and direct the beam through the specimen.
- Image Formation Process
The basic mechanism of image formation remains consistent across both techniques. As electrons pass through the specimen, they undergo various interactions including scattering and absorption. These interactions create contrast in the final image, with the transmitted electrons being collected and focused by objective lenses to form the image.
Key Technological Distinctions
- Beam Energy and Dose Control
While TEM typically operates with higher electron doses suitable for fixed specimens, Cryo-EM requires sophisticated dose control systems. In Cryo-EM, the electron dose must be carefully managed to prevent radiation damage to sensitive biological samples, often utilizing specialized low-dose imaging techniques.
- Detection Systems
Modern Cryo-EM employs direct electron detectors capable of recording multiple frames per second, enabling motion correction and improved signal-to-noise ratios. Traditional TEM often uses more conventional CCD cameras or phosphor screens, which are suitable for the higher contrast images produced by stained specimens.
- Focus and Alignment Systems
Cryo-EM systems incorporate advanced automated focusing and alignment mechanisms to maintain sample integrity during minimal exposure times. TEM systems, while also sophisticated, can employ more straightforward focusing procedures due to the stability of chemically fixed samples.
Resolution Capabilities and Limitations
- Resolution Determinants
The achievable resolution in both techniques depends on multiple factors. These include the electron optical system quality, mechanical stability, and specimen characteristics. Cryo-EM has recently achieved breakthrough resolutions approaching 1.5Å for some specimens, while TEM typically provides resolutions suitable for cellular ultrastructure analysis.
- Technical Limitations
Each method faces distinct challenges affecting resolution. For TEM, these primarily relate to sample preparation artifacts and staining effects. Cryo-EM confronts challenges with beam-induced motion, radiation damage, and the inherent contrast limitations of unstained specimens in ice.
- Sample-Specific Considerations
Resolution capabilities vary significantly depending on the specimen type. While Cryo-EM excels in resolving molecular structures of purified proteins and complexes, TEM often provides better contrast for cellular components and materials science applications where chemical fixation is acceptable.
Optimal Applications of TEM and Cryo-EM Technologies
Structural Biology Applications
| Protein Structure Determination | Cryo-EM has revolutionized the field by enabling direct visualization of protein complexes in their native states, particularly valuable for large assemblies that resist crystallization. The technique excels at resolving dynamic regions and capturing multiple conformational states, providing crucial insights into protein function and regulation. |
| Drug-Target Interactions | Cryo-EM's ability to visualize drug binding sites at near-atomic resolution has become invaluable for structure-based drug design. By capturing various conformational states of drug-protein complexes, researchers can optimize drug candidates and understand resistance mechanisms. |
| Membrane Proteins | The study of membrane proteins represents a particular strength of Cryo-EM. These challenging targets, often resistant to traditional crystallographic methods, can be studied in lipid environments that better mimic their natural state. This capability has led to breakthroughs in understanding ion channels, transporters, and receptor signaling mechanisms. |
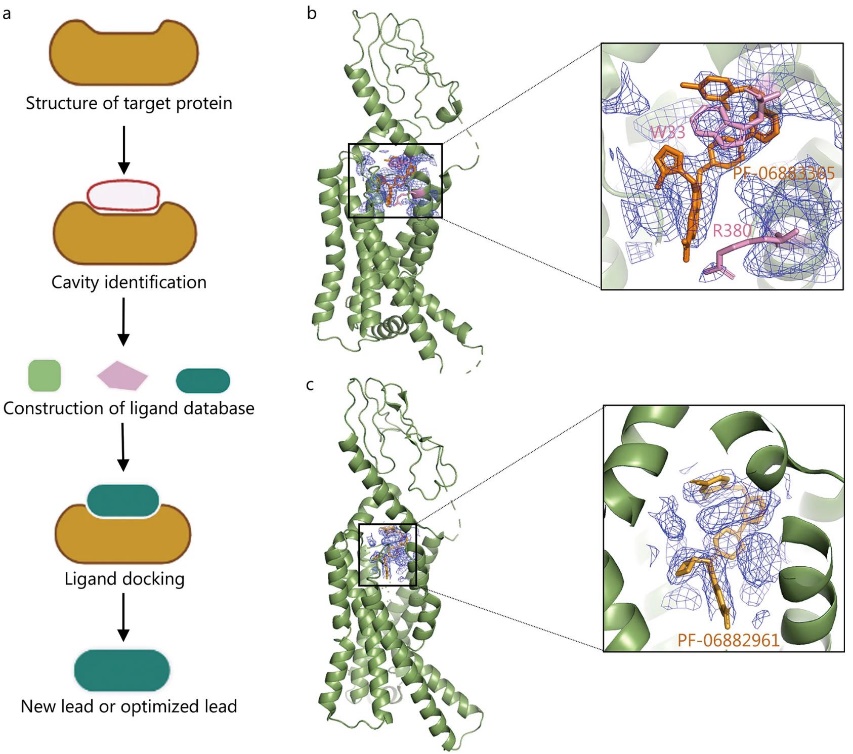 Figure 2. Cryo-EM applications in structure-based drug design. Illustration of cryo-EM in structure-based drug design (SBDD). (a) Schematic of SBDD technology. (b, c) Cryo-EM structures of GLP-1R bound with small molecules PF-06883365 and PF-06882961, resolved at 3.8 Å and 2.5 Å, respectively. The structures highlight ligand density and key binding residues. (Zhu K F, et al., 2023)
Figure 2. Cryo-EM applications in structure-based drug design. Illustration of cryo-EM in structure-based drug design (SBDD). (a) Schematic of SBDD technology. (b, c) Cryo-EM structures of GLP-1R bound with small molecules PF-06883365 and PF-06882961, resolved at 3.8 Å and 2.5 Å, respectively. The structures highlight ligand density and key binding residues. (Zhu K F, et al., 2023)
Cell Biology Applications
| Cellular Ultrastructure | TEM remains the gold standard for cellular ultrastructure studies. Its ability to provide high-contrast images of fixed specimens allows detailed examination of cell architecture, including membrane systems, cytoskeletal elements, and organelle distribution. This approach is particularly valuable for understanding cellular organization and pathological changes. |
| Organelle Visualization | Both techniques offer unique perspectives on organelle structure and function. While TEM provides excellent contrast for examining organelle morphology and distribution, Cryo-EM enables visualization of native protein complexes within cellular contexts, bridging the gap between structural and cellular biology. |
| Dynamic Processes | Recent advances in both technologies have enhanced our ability to study cellular dynamics. Time-resolved Cryo-EM can capture molecular machines in action, while specialized TEM techniques allow visualization of cellular processes under various conditions, providing insights into cell division, trafficking, and stress responses. |
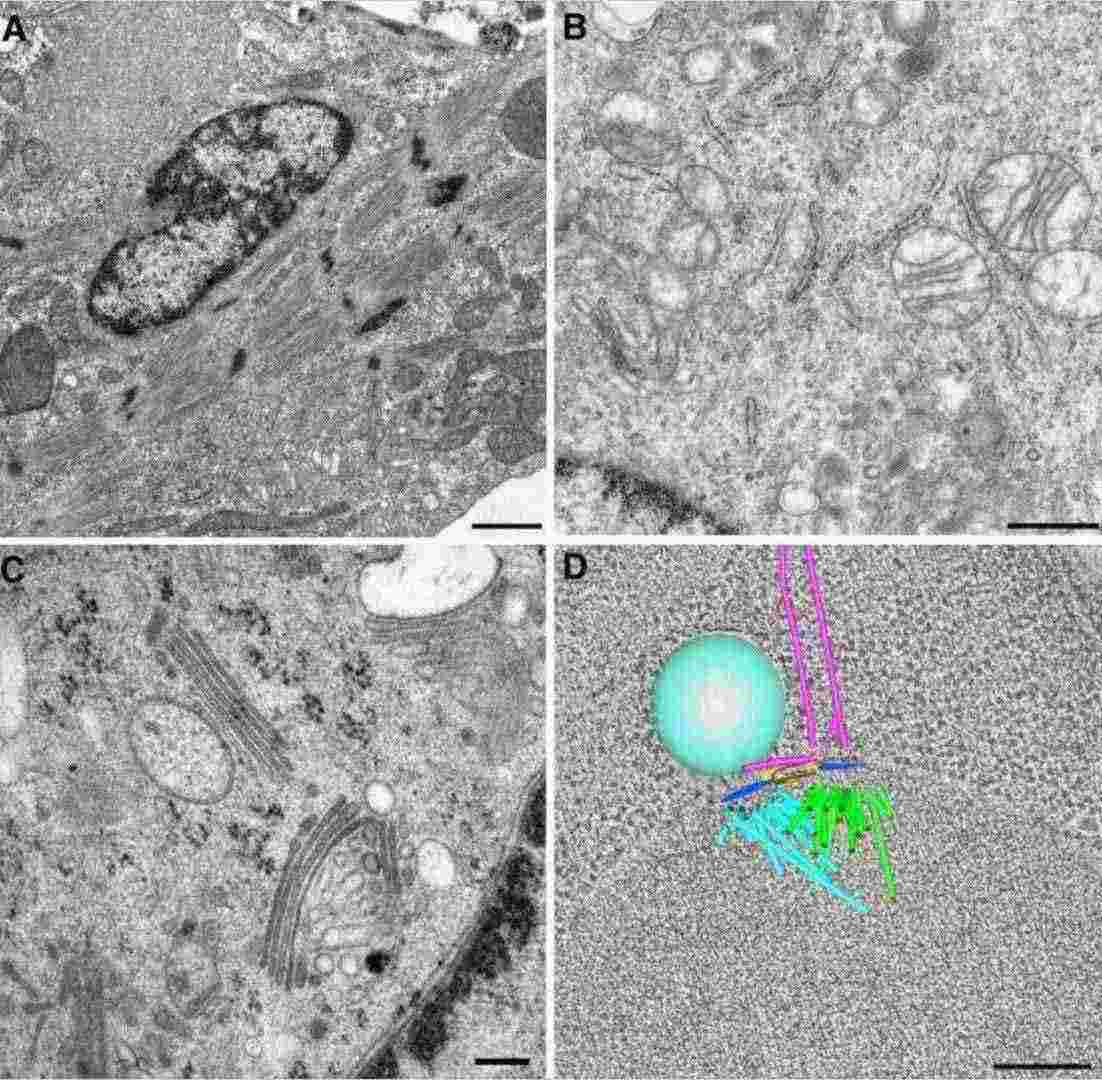 Figure 3. Cellular ultrastructure visualized by transmission electron microscopy. TEM images showing (A) the actin-myosin cytoskeleton in a cardiomyocyte, (B) organelles in a mouse macrophage, (C) Golgi membranes in a 3T3 cell using high-pressure freezing, and (D) a 3D tomographic model of a forming mitotic spindle in budding yeast. (Winey M, et al., 2014)
Figure 3. Cellular ultrastructure visualized by transmission electron microscopy. TEM images showing (A) the actin-myosin cytoskeleton in a cardiomyocyte, (B) organelles in a mouse macrophage, (C) Golgi membranes in a 3T3 cell using high-pressure freezing, and (D) a 3D tomographic model of a forming mitotic spindle in budding yeast. (Winey M, et al., 2014)
Materials Science Applications
| Nanostructure Analysis | In materials science, both techniques excel at different aspects of nanostructure characterization. TEM provides atomic-resolution imaging of crystal lattices and defect structures, while Cryo-EM offers unique capabilities for studying soft materials and organic-inorganic interfaces under preserved conditions. |
| Surface Characterization | Surface analysis capabilities differ between the techniques. TEM excels at analyzing surface modifications and coatings at high resolution, particularly valuable for engineered materials. Cryo-EM offers advantages for studying surface interactions in their native state, especially important for bio-interface materials. |
| Material Properties | The complementary nature of these techniques enables comprehensive material property analysis. TEM provides detailed information about crystallographic structure and compositional distribution, while Cryo-EM allows examination of soft materials and complex composite systems under near-native conditions. This combination is particularly powerful for developing new materials with biological applications. |
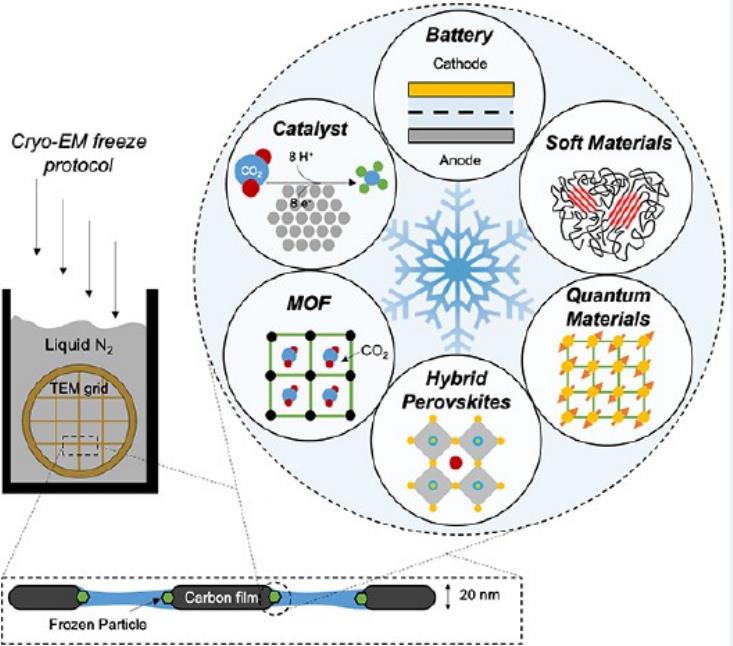 Figure 4. Applications of cryo-EM in materials science. Six key areas in materials science that can benefit from cryo-EM: batteries, soft polymers, metal-organic frameworks, perovskite solar cells, electrocatalysts, and quantum materials. (Li Y, et al., 2020)
Figure 4. Applications of cryo-EM in materials science. Six key areas in materials science that can benefit from cryo-EM: batteries, soft polymers, metal-organic frameworks, perovskite solar cells, electrocatalysts, and quantum materials. (Li Y, et al., 2020)
Select Service
Related Reading
Strategic Selection Guide: TEM vs Cryo-EM Technology
Sample Type Assessment
The nature of your sample fundamentally directs your choice between TEM and Cryo-EM. Biological specimens requiring native state preservation are better suited for Cryo-EM, while materials science samples or fixed biological specimens often achieve optimal results with conventional TEM. Consider factors such as sample stability, size range, and structural complexity.
Research Objectives Alignment
Your research goals play a crucial role in technology selection. High-resolution protein structure determination demands Cryo-EM capabilities, while cellular ultrastructure studies might be better served by TEM. Consider whether you need atomic-level resolution or if cellular-level details suffice for your research questions.
Technical Requirements Analysis
Each technology demands specific technical capabilities. Evaluate factors such as required resolution, contrast needs, and data collection requirements. Consider whether your research requires specialized features like time-resolved imaging or in-situ observations.
Operational Timeline Planning
Project timelines influence technology selection. TEM typically offers faster sample preparation and data collection, while Cryo-EM projects often require longer timeframes for optimization and data processing. Consider your research deadlines and publication goals.
Sample Management Strategy
Evaluate your sample availability and preparation requirements. Cryo-EM often requires less sample quantity but demands higher sample purity. TEM may require more sample material but offers more forgiving preparation protocols.
Understanding the distinct capabilities and applications of Cryo-EM and TEM is crucial for advancing research in structural biology, cell biology, and materials science. At Creative Biostructure, we offer comprehensive Cryo-EM services and TEM services. Our team of experts can help you select the most suitable technique for your specific research needs and guide you through the entire process from sample preparation to data analysis. Contact us to discuss your project requirements and discover how our electron microscopy services can accelerate your research goals.
References
- Nagashima K, Zheng J, Parmiter D, et al. Biological tissue and cell culture specimen preparation for TEM nanoparticle characterization. Characterization of Nanoparticles Intended for Drug Delivery. 2011: 83-91.
- Winey M, Meehl J B, O'Toole E T, et al. Conventional transmission electron microscopy. Molecular Biology of the Cell. 2014, 25(3): 319-323.
- Callaway E. Revolutionary cryo-EM is taking over structural biology. Nature. 2020, 578(7794): 201-202.
- Li Y, Huang W, Li Y, et al. Opportunities for cryogenic electron microscopy in materials science and nanoscience. ACS nano. 2020, 14(8): 9263-9276.
- Zhu K F, Yuan C, Du Y M, et al. Applications and prospects of cryo-EM in drug discovery. Military Medical Research. 2023, 10(1): 10.

-1.jpg)