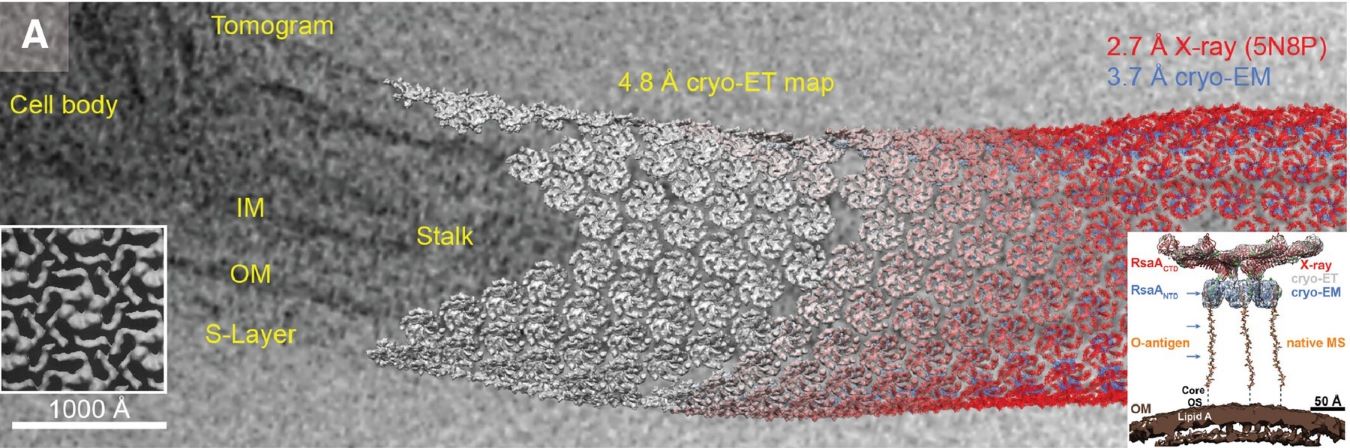
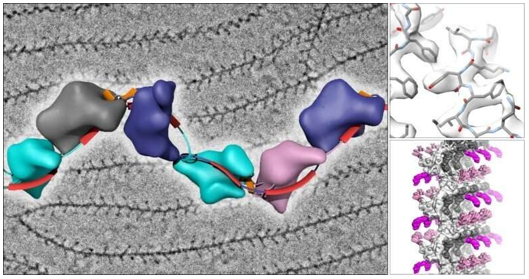
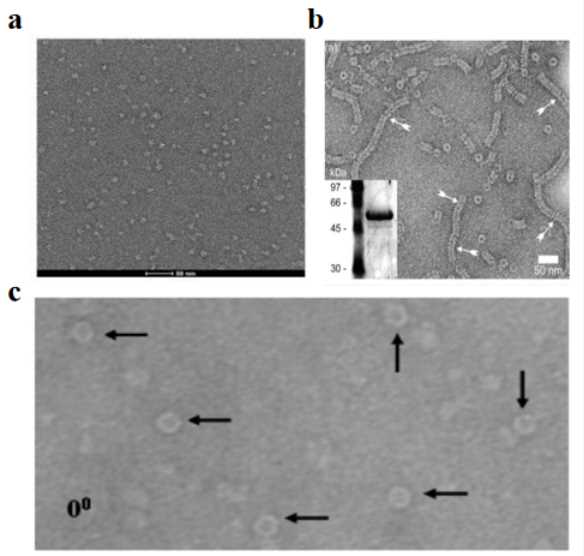
What Is Negative Stain Electron Microscopy
Negative staining electron microscopy (EM) represents a fundamental yet powerful technique in structural biology, offering rapid visualization of biological specimens at nanometer resolution. In this approach, biological samples are embedded in a thin layer of heavy metal stains such as uranyl acetate or phosphotungstic acid, creating a high-contrast "negative" image where the specimen appears as a light region against a dark background. Since its development in the 1950s, negative staining EM has evolved into an indispensable tool for preliminary sample screening and quality assessment prior to advanced cryo-EM studies. Its versatility extends across multiple fields, from analyzing protein complexes and virus particles to characterizing nanoparticles and macromolecular assemblies. While modern cryo-EM achieves atomic resolution, negative staining remains crucial for rapid sample evaluation, offering invaluable insights into sample heterogeneity, stability, and overall structural features that guide subsequent high-resolution studies.
Key Highlights:
- Negative staining electron microscopy provides rapid, high-contrast imaging of biological specimens
- Essential screening tool before proceeding to expensive cryo-EM studies
- Resolution typically ranges from 15-20 Å
- Widely used in structural biology for protein complexes, viruses, and nanoparticles
Negative Stain Techniques and Methodologies
A. Traditional Negative Staining
The traditional negative staining process involves depositing biological samples onto a support film, typically carbon-coated grids, followed by application of electron-dense staining agents. The most widely used stains include uranyl acetate (UA) and uranyl formate (UF), which provide excellent contrast due to their high atomic numbers. The staining process involves careful optimization of parameters such as stain concentration (typically 0.5-2%), staining time (30-60 seconds), and blotting conditions. This technique offers rapid sample screening with minimal specimen preparation, enabling visualization of structural features down to 15-20 Å resolution. However, limitations include potential staining artifacts, sample flattening, and limited preservation of native structural features.
B. Cryo-Negative Staining
Cryo-negative staining combines elements of traditional negative staining with cryo-preservation techniques, offering enhanced structural preservation. In this method, samples are stained and rapidly vitrified in liquid ethane, maintaining them in a partially hydrated state. This approach provides several advantages over traditional negative staining, including better preservation of delicate structural features and reduced staining artifacts. Critical factors affecting resolution and contrast include stain thickness, ice quality, and specimen temperature maintenance. The technique particularly excels in visualizing membrane proteins and larger macromolecular complexes, achieving resolutions around 10-15 Å.
C. Negative Stain for High-Resolution Cryo-EM
Negative staining serves as an essential preliminary screening tool in the cryo-EM workflow, enabling rapid assessment of sample quality and heterogeneity before proceeding to more resource-intensive cryo-EM studies. This integration significantly enhances workflow efficiency by identifying optimal specimen conditions and concentrations. For instance, in structural studies of membrane proteins or virus particles, negative staining can quickly reveal particle distribution and aggregation states, guiding subsequent cryo-EM grid preparation parameters. This combined approach has proven particularly valuable in recent structural studies of challenging targets such as membrane protein complexes, where initial negative staining assessment dramatically improves the success rate of subsequent high-resolution cryo-EM analysis.
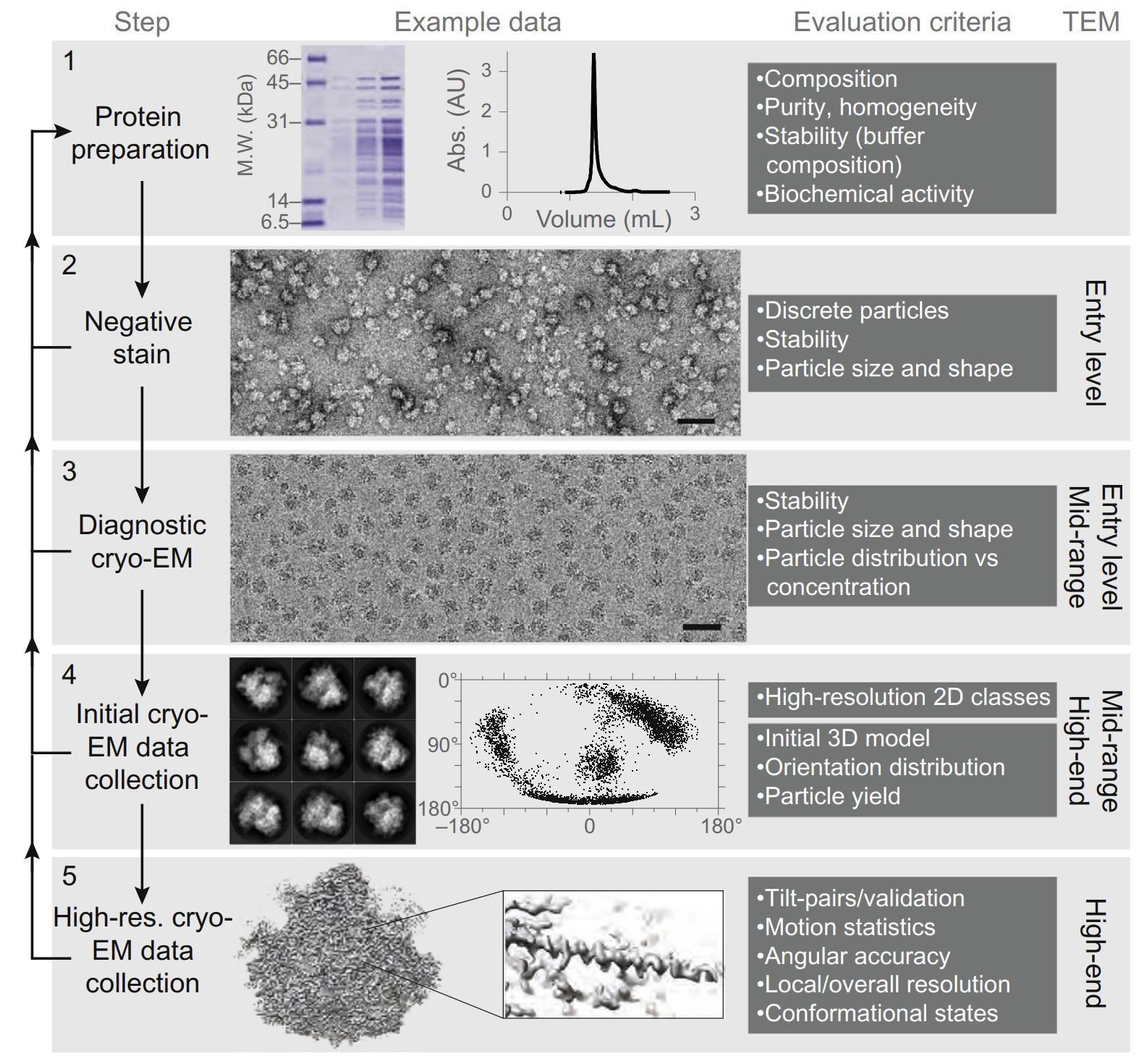 Figure 1. Role of negative staining in the cryo-EM workflow for 3D structure determination. (Passmore L A, et al., 2016)
Figure 1. Role of negative staining in the cryo-EM workflow for 3D structure determination. (Passmore L A, et al., 2016)
Comparative Analysis: Negative Staining vs Cryo EM
A. Resolution and Contrast
In structural biology, resolution capabilities represent a critical distinction between negative staining and cryo-EM techniques. Negative staining typically achieves resolutions around 15-20 Å, limited by grain size of the heavy metal stains and potential staining artifacts. In contrast, modern cryo-EM can routinely achieve near-atomic resolutions below 3 Å, enabling visualization of amino acid side chains and secondary structural elements. However, negative staining provides superior contrast due to the electron-dense stain, resulting in higher signal-to-noise ratios that facilitate easier particle detection and initial model generation, particularly beneficial for smaller proteins (<100 kDa) or low-abundance samples.
B. Sample Preparation and Screening
Sample preparation workflows differ significantly between these techniques. Negative staining offers rapid sample assessment within 15-30 minutes, making it ideal for preliminary screening and optimization. The procedure requires minimal specialized equipment and can be performed at room temperature. In contrast, cryo-EM sample preparation demands sophisticated vitrification equipment, precise temperature control, and significantly more expertise. While negative staining may alter native protein conformations due to dehydration and stain interactions, cryo-EM preserves specimens in a near-native state through vitrification, maintaining structural integrity and hydration shell critical for biological function.
Select Service
C. Practical Applications
Each technique has its optimal application scenarios based on research objectives and sample characteristics. Negative staining excels in rapid sample screening, quality assessment of protein preparations, antibody labeling studies, and initial structural characterization of novel complexes. It's particularly valuable for heterogeneous samples, membrane proteins during purification optimization, and initial evaluation of dynamic complexes. Cryo-EM becomes the method of choice when pursuing high-resolution structural details, studying flexible proteins in multiple conformational states, analyzing membrane proteins in lipid environments, and investigating complex molecular machines in their native states. The complementary use of both techniques often provides the most comprehensive structural insights, with negative staining guiding optimal conditions for subsequent high-resolution cryo-EM analysis.
| Aspect | Negative Staining TEM | Cryo-TEM |
| Resolution |
|
|
| Contrast |
|
|
| Sample Preparation |
|
|
| Sample State |
|
|
| Best Applications |
|
|
| Cost & Time |
|
|
| Equipment Requirements |
|
|
| Limitations |
|
|
Practical Tips and Best Practices
A. Optimizing Negative Staining
- Stain Properties: The choice and preparation of stain significantly impact image quality. Use freshly prepared uranyl acetate at 1% w/v concentration (range 0.5-2%), filtered through a 0.22 μm filter and stored protected from light at 4°C.
- Sample Preparation: Optimize protein concentration between 0.01-0.1 mg/mL in a stain-compatible buffer to achieve ideal particle distribution and prevent aggregation.
- Grid Treatment: Perform glow discharge or plasma cleaning immediately before use to ensure optimal surface hydrophilicity and sample spreading on the carbon support film.
- Staining Process: Maintain staining time between 30-60 seconds under controlled humidity (50-70% RH), using proper blotting technique with filter paper at a 45° angle for even stain distribution.
- Environmental Control: Conduct all procedures at controlled room temperature (20-25°C) with proper humidity, ensuring clean and dust-free workspace conditions.
B. Preparing for Cryo-EM
Negative staining serves as an essential quality control step before proceeding to cryo-EM. Begin by evaluating sample homogeneity and concentration using multiple negative stain grids. Check for proper particle distribution (ideally 100-200 particles per field of view), absence of aggregation, and consistent particle shapes. Assess protein stability over time by preparing grids at different time points. Use this preliminary data to optimize buffer conditions, particularly important for membrane proteins. Document the particle size distribution and any preferred orientations, as this information guides cryo-EM grid preparation parameters. If heterogeneity is observed, consider additional purification steps before proceeding to cryo-EM.
C. Troubleshooting Common Issues
| Problem | Possible Causes | Solutions |
| Sample Aggregation |
|
|
| Poor Contrast |
|
|
| Uneven Stain Distribution |
|
|
| Preferred Orientation |
|
|
| Staining Artifacts |
|
|
(Note: UA = Uranyl Acetate, RH = Relative Humidity)
Negative staining and cryo-EM represent complementary techniques in modern structural biology, each offering distinct advantages for specific research applications. While negative staining provides rapid, high-contrast imaging crucial for initial sample screening and quality assessment, cryo-EM enables breakthrough atomic-resolution studies of biological specimens in their native states. As structural biology advances, negative staining will remain an indispensable tool in the preliminary stages of structural studies, working synergistically with cryo-EM to accelerate discoveries in life sciences and drug development.
Creative Biostructure provides comprehensive electron microscopy services, including both negative staining TEM service and cryo-EM services. Our experienced team offers customized solutions for your structural biology needs, from initial sample screening to high-resolution structure determination. Whether you need rapid sample assessment through negative staining or atomic-resolution structures via cryo-EM, our state-of-the-art facilities and expert scientists are ready to support your research goals. Contact us to discuss your project requirements and discover how our EM services can accelerate your research.
References
- Harris J R, De Carlo S. Negative staining and cryo-negative staining: applications in biology and medicine. Electron Microscopy: Methods and Protocols. 2014: 215-258.
- Passmore L A, Russo C J. Specimen preparation for high-resolution cryo-EM. Methods in enzymology. 2016, 579: 51-86.
- Gallagher J R, Kim A J, Gulati N M, et al. Negative‐stain transmission electron microscopy of molecular complexes for image analysis by 2D class averaging. Current protocols in microbiology. 2019, 54(1): e90.
- Gonen S. Progress towards cryoEM: negative-stain procedures for biological samples. cryoEM: Methods and Protocols. 2021: 115-123.
- Nguyen V, Somavarapu A K, Sharma R G, et al. A rapid automated system for negative staining EM. Biophysical Journal. 2024, 123(3): 132a-133a.

-1.jpg)