Liposome Stability Analysis
Creative Biostructure has accumulated rich experience in the field of liposome research & development through hundreds of successful scientific projects in recent years. Integrated with other advanced platforms, Creative Biostructure is able to provide the most comprehensive products and services including liposome stability analysis.
Why stability analysis is important to liposome?
When liposomes are used as drug carriers, their stability during storage or processing in human body are two major concerns in liposome design and application. The former is crucial to liposomes in food applications, while the latter is extremely vital to liposomes in pharmaceutical applications. There are lots of factors affecting the structural and functional stability, including temperature, pH and the presence of degradation substances. Therefore, functional liposomes must be proved to have adequate stability in consideration of both chemical and physical changes.
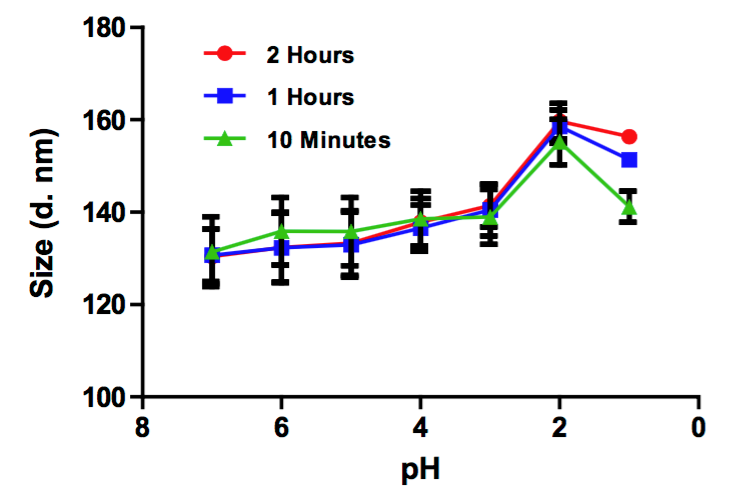 Figure 1. Liposome encapsulated DHA was exposed to various pHs from 1 to 7.4 for 10 min, one hour, and two hours. Dynamic light scattering (DLS) was performed at every hour and at various pHs. All diameters are presented in nanometers. Though the size of liposome increased to 156.4 ± 0.3 nm at pH 1, the size of liposome was still below 200 nm, indicating the liposomal DHA is ideal for the uptake and transfer in stomach. (C. G. Skibinski, et al., 2016)
Figure 1. Liposome encapsulated DHA was exposed to various pHs from 1 to 7.4 for 10 min, one hour, and two hours. Dynamic light scattering (DLS) was performed at every hour and at various pHs. All diameters are presented in nanometers. Though the size of liposome increased to 156.4 ± 0.3 nm at pH 1, the size of liposome was still below 200 nm, indicating the liposomal DHA is ideal for the uptake and transfer in stomach. (C. G. Skibinski, et al., 2016)
Liposome stability analysis methods
Physical stability
The physical stability of liposome can be affected by a number of factors such as the liposome integrity, size distribution, unsaturated fatty acid groups. Defects in lattice structure can lead to liposome fusion, aggregation and leakage of contained drug substance during storage. Therefore, stability testing should include tests to assess liposome size distribution and integrity. Analytical methods based on fluorescence changes in FRET and epifluorescence microscopy are especially useful for measuring the physical stability of liposomes. Freezing, spray-drying and freeze-drying might also be considered in order to guarantee the long-term stability of liposome. Furthermore, the ability of liposomes to stay intact during drug transportation is the core value of any liposomes, and should be measured properly in prior to applications.
Chemical stability
The evaluation the chemical stability includes the lipid components in the liposome as well as the chemical stability of the contained drug substance. Degradation of natural phospholipids occurs mainly in two ways, one is the peroxidation of double bonds present in acyl chains, the other one is the hydrolysis of ester bonds linking fatty acids to the glycerol moiety. Usage of high quality lipids, suitable preparation method and storage condition can all help minimize the degradation of liposome through peroxidation. To reduce the oxidation rate, liposomes must be kept from light and oxygen. There are a lot of methods can be used to monitor the degradation of lipids. For example, thin layer chromatography (TLC) can be applied to measure the purity and concentration of the lipids. In terms of chemical stability of loaded drug, pharmacodynamics test could be re-conducted to confirm clinical efficiency.
Creative Biostructure is an industry-leading provider for high-quality products, assays and custom services. Our programs and service packages are offered at highly competitive prices with rapid turnaround time. Please contact us to share more about your research and get a detailed quote.
Ordering Process
References
- C. G. Skibinski, et al. (2016). A novel biologically active acid stable liposomal formulation of docosahexaenoic acid in human breast cancer cell lines. Chemico-Biological Interactions, 256: 1-8.
- M. Marsanasco, et al. (2015). Bioactive constituents in liposomes incorporated in orange juice as new functional food: thermal stability, rheological and organoleptic properties. J. Food Sci. Technol., 52(12): 7828–7838.
- J. F. Boelter and A Brandelli. (2016). Innovative bionanocomposite films of edible proteins containing liposome-encapsulated nisin and halloysite nanoclay. Colloids and Surfaces B: Biointerfaces, 145: 740-747.
