Mempro™ Multivesicular Vesicles Liposomes
Creative Biostructure is a specialty company focused on providing protein production and structural biology research, development and manufacturing expertise in the production of membrane proteins, also known as liposomes, to clients and partners in both the academic and industrial sources. We have built our advanced Mempro™ Liposome Technology to provide all classified types of liposomes, such as Multivesicular Liposomes/Vesicles (MVVs).
Liposomes have shown great potential as versatile drug delivery systems to deliver numerous bioactives. These include proteins, antineoplastic agents, antibiotics, and antiviral drugs. Liposome formulations for interferons, peptides, and hormones (calcitonin) have successfully been used as intramuscular depots. Recently attention in liposome preparation technology has been focused on the preparation of liposomes with a large number of bilayers.
However, the conventional liposomes (unilamellar and multilamellar liposomes) have certain limitations with low entrapment efficiency for water-soluble drugs, stability problems, and release of drugs after a single breach in the external membrane. Scientists have made great effort to overcome the limitations of conventional therapies and to investigate in vivo effectiveness for sustained delivery. MVLs of acyclovir were prepared by the reverse phase evaporation method. The loading efficiency of the MVLs (45%-82%) was found to be 3 to 6 times higher than conventional multilamellar vesicles (MLVs).
This challenge has been successfully met by the use of a new carrier, ie, a multivesicular liposomal (MVL) drug delivery system. The MVL systems are characterized by their sui-generis structure of multiple nonconcentric aqueous chambers surrounded by a network of lipoidal membranes. These systems can be differentiated from conventional liposomes (Unilamellar Vesicles [ULVs] and multilamellar vesicles [MLVs]) in 2 major aspects: size and composition. MVL carriers have a size range of 5 to 30 μm compared with ULVs (1 μm) and MLVs (1-5 μm).
The structural characteristics of MVLs make them ideal vehicles for locoregional drug delivery. The MVL particles are manufactured with lipids, which are a membrane tissue component of the human body, hence they are highly bio- compatible and nonimmunogenic. Their unique particle size is large enough to preclude their entry into the capillaries and thus remain at the site of administration. Since most of the volume of MVLs is occupied by water, lipid remnants are minimal once the contents are released.
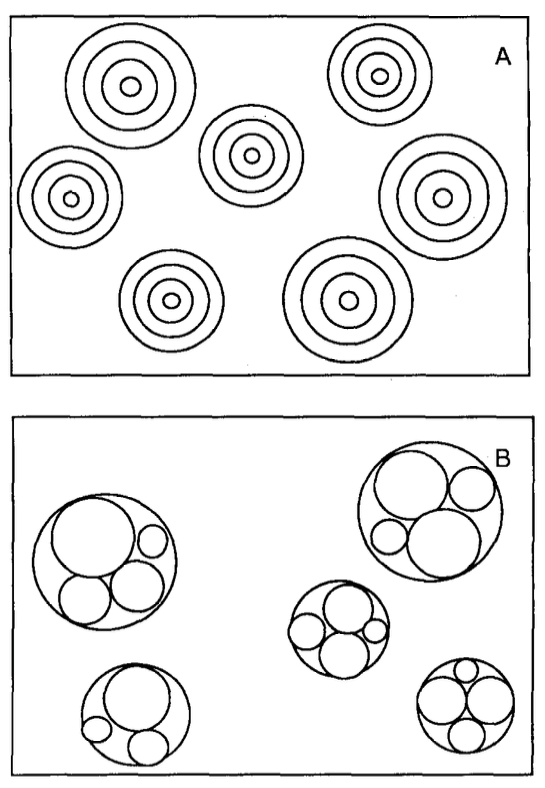 Figure 1. Types of multilamellar vesicles. A: Classical multilamellar vesicles. B: Multivesicular vesicles.
Figure 1. Types of multilamellar vesicles. A: Classical multilamellar vesicles. B: Multivesicular vesicles.
Besides LUVs, we also provide other sizes of liposomes, such as Small Unilamellar Vesicles (SUV), Large Unilamellar Vesicles (LUV)and Giant Unilamellar Vesicles (GUV), and respective analysis service.
Reference:
Sanjay K. Jain et al. Design and Development of Multivesicular Liposomal Depot Delivery System for Controlled Systemic Delivery of Acyclovir Sodium. AAPS PharmSciTech 2005; 6 (1) Article 8
H. Talsma et al. Multilamellar or multivesicular vesicles? International Journal of Pharmaceutics, 37 (1987) 171-173