MemPro™ lysosome-associated membrane glycoprotein
Creative Biostructure provides custom gene-to-structure services for Lysosome-associated membrane glycoprotein.
Lysosome-associated membrane glycoprotein (LAMP) is a type I single pass integral membrane protein which is highly expressed in skeleton muscles and placenta. LAMP is abundant proteins, representing 0.1 to 0.2% of total cell protein and has two closely related types: LAMP1 (CD107a) and LAMP2 (CD107b). They are among the most extensively glycosylated proteins with glycan chains that outweigh the protein core and that include high-molecular-weight poly-N-acetyllactosaminoglycans. The transmembrane region is followed by a short, C-terminal cytosolic tail that comprises motifs for lysosomal targeting. LAMP-1 and 2 are major components of the glycoconjugate coat on the inside of the lysosomal membrane. It was estimated that the concentration of LAMPs is sufficiently high for the formation of a nearly continuous layer on the inner surface of the lysosomal membrane. They are believed to share similar functions in maintaining lysosomal integrity, pH and catabolism.
LAMP knockout mice and cell line have provided significant evidence about LAMP functions. Double LAMP-1/2 knockout leads to embryonic lethality, however LAMP-1 knockout mice are not impaired while displaying upregulated levels of LAMP-2. It proves that LAMP-1 and 2 can complement each other to a large extent. Knockout of the LAMP-2 gene leads to elevated postnatal mortality Surviving mice have a phenotype that corresponds to Danon disease, a rare genetic condition caused by LAMP-2 deficiency which is characterized by the abnormal accumulation of glycogen in muscles.
Chaperone-mediated autophagy (CMA) is a highly regulated cellular process that mediates selective removal of impaired cytosolic proteins. LAMP-2 isoform LAMP-2A, functions as a receptor for cytosolic proteins, is key regulators of CMA. LAMP-2A exists as a homotrimer in which the helices wrap around each other to form a parallel coiled coil structure. This distinct secondary structure interacts with chaperone and CMA substrate simultaneously to propose a substrate recognition.
Intracellular glycoprotein coats are believed to protect the limiting membranes of degradative vesicles against acid hydrolases. CD107a is significantly upregulated on the surface of NK cells following stimulation with MHC devoid targets. The expression levels of CD107a is correlated with NK cells cytotoxic activity. Cell surface expression of LAMP (CD107) is often observed in tumor cells, especially metastatic cancer. They are also involved in cell selection and help mediate cell-cell adhension.
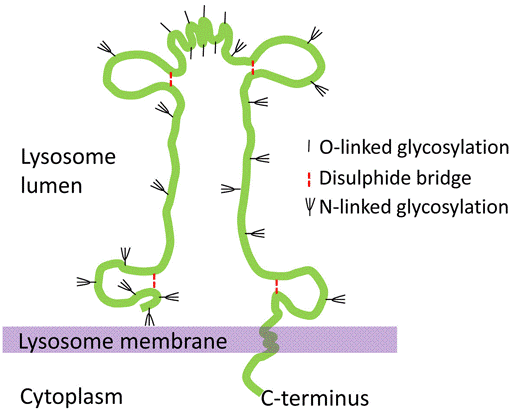
Figure 1. Molecular structure of LAMP in lysosome membrane.
References:
Wilke S, Krausze J, Büssow K. Crystal structure of the conserved domain of the DC lysosomal associated membrane protein: implications for the lysosomal glycocalyx[J]. BMC biology, 2012, 10(1): 1.
Rout A K, Strub M P, Piszczek G, et al. Structure of transmembrane domain of lysosome-associated membrane protein type 2a (LAMP-2A) reveals key features for substrate specificity in chaperone-mediated autophagy[J]. Journal of Biological Chemistry, 2014, 289(51): 35111-35123.