Custom MemPro™ Stannin (SNN) Membrane Proteins Services
Creative Biostructure has developed custom MemPro™ gene-to-structure services for Stannin (SNN) membrane proteins. SNN is a highly conserved, 88-amino acid membrane protein. It was firstly isolated from neuronal cells sensitive to trimethyltin (TMT).Researches have revealed SNN locates in multiple tissue regions, including neuron, lymph, spleen, renal, kidney and lung.
Solution and solid-state NMR can be used to elucidate the structure and dynamics of SNN. This protein consists of three domains: a single transmembrane helix, an unstructured linker and a distorted cytoplasmic helix. Understanding of SNNs’ structures can facilitate the study of its role in organotin toxicity, cellular growth, apoptosis and mitochondrial response to injury.
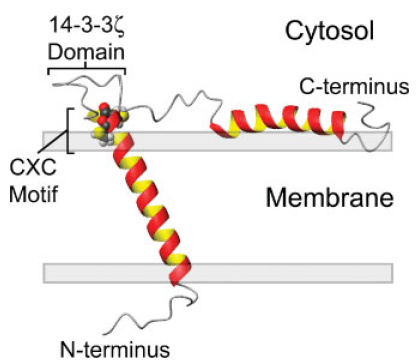 Figure. Model of SNN oriented in a lipid bilayer highlighting the CXC alkyltin metal binding motif residing at the lipid/solvent interface (residues Cys-32 and Cys-34) and the putative 14-3-3 ζ binding domain between residues 39 and 45.
Figure. Model of SNN oriented in a lipid bilayer highlighting the CXC alkyltin metal binding motif residing at the lipid/solvent interface (residues Cys-32 and Cys-34) and the putative 14-3-3 ζ binding domain between residues 39 and 45.
Stannin was initially isolated from neuronal cells damaged by exposure to TMT (trimethyltin). It is expressed by multiple tissue regions including spleen, kidney, lymph, striatum, neuronal tissues, cerebellum, midbrain, hippocampus neocortex, etc. Just as shown in the figure, SNN is consisted by a single transmembrane helix (residues 10–33), a cytoplasmic helix (residues 61–79, partially embedded into the membrane), and a linker region (residues 34–60) seperating the two helixes. The 28-residue linker region contains a conserved metal binding CXC motif located at the lipid/solvent interface and putative 14-3-3 binding region located distally to the CXC motif. The location of CXC motif facilitates it to interact with organotin compounds. Many experiments have revealed that SNN prefers to be located in the mitochondrial membrane of neuronal cells and it is considered to be responsible for the sensitivity of various cells to TMT toxicity. Additionally, SNN also plays crucial roles in activating several key signal systems, such as p53-dependent pathways, p38-ERK cascade, and 14-3-3 protein-mediated processes. Decreased SNN expression reduces the growth of endothelial cells and induction of COP-1, which suggests that SNN may be involved in growth regulation.
Creative Biostructure can provide custom MemPro™ gene-to-structure services for membrane proteins. Please click for more information.
M.L. Billingsley, et al. Functional and structural properties of stannin: roles in cellular growth, selective toxicity, and mitochondrial responses to injury. Journal of Cellular Biochemistry. 2006 May 15; 98 (2):243-50.
Bethany A. Buck-Koehntop, et al. Structure, dynamics, and membrane topology of stannin: a mediator of neuromal cell apoptosis induced by trimethyltin chloride. Journal of molecular Biology. 2005 Dec 2; 354 (3): 652-65.
