Custom MemPro™ Peptidase Family M48 Services
Creative Biostructure provides custom MemPro™ gene-to-structure services for Peptidase Family M48. Our expert scientists can offer you E. coli strains, expression vectors, detergents, multiple overexpression and purification strategies for high-yield production of stable and functional membrane proteins.
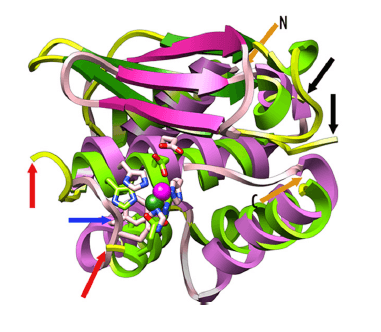 Figure 1. A model of minigluzincins structure. (J. Biol. Chem., 2013)
Figure 1. A model of minigluzincins structure. (J. Biol. Chem., 2013)
Peptidase Family M48 (also termed Ste24 endopeptidase family) mainly contains metalloendopeptidases, which are integral membrane proteins associated with endoplasmic reticulum and Golgi. Peptidase Family M48 can be divided into three subtypes: M48A (Prototype is Saccharomyces cerevisiae Ste24p), M48B (Prototype is Escherichia coli HtpX), and M48C (Prototype is human mitochondrial Oma1 peptidase). These peptidases in family M48 possess a single catalytic zinc ion coordinated by two histidines within an HEXXH motif, which is extracellular but adjacent to a transmembrane domain and therefore close to the membrane surface. The glutamate within the HEXXH motif is a catalytic residue. Site-directed mutagenesis of the His and Glu residues within the HEXXH motif of Ste24 endopeptidase, which is an integral membrane protein with seven transmembrane domains, has confirmed that these are essential for catalysis. The homologues of peptidase Family M48 have been found in animals, plants and bacteria. Eukaryotic peptidase family M48 has a requirement for substrates that are bound at a C-terminal motif known as CAAX, in which A is an aliphatic residue, and the lipid is attached to the cysteine residue. With every subunit binding one zinc ion, metalloendopeptidases can cleave misfolded proteins and inhibit their accumulation in the membrane. Yeast Ste24 endopeptidase is essential for the processing of a-mating factor precursor. In bacteria, the HtpX endopeptidases are induced by heat shock and can be involved in the process of abnormal protein degradation. Human Oma1 is localized in the inner membrane of mitochondria and plays a role in the quality control system.
In addition to the recombinant overexpression and purification of membrane proteins, Creative Biostructure can also determine the crystal structures of Peptidase family M48 by multiply approachestailored to your specific requirements.
Creative Biostructure has extensive experience in the overexpression, purification and characterization of membrane proteins. Creative Biostructure can provide custom MemPro™ membrane protein gene-to-structure services. Please feel free to contact us for a detailed quote.
References:
K. Fujimura-Kamada, et al. (1997). A novel
membrane-associated metalloprotease, Ste24p, is required for the first step of
NH2-terminal processing of the yeast a-factor precursor. J. Cell Biol., 136: 271-285.
M. López-Pelegrín et al. (2013). A novel family of soluble
minimal scaffolds provides structural insight into the catalytic domains of
integral membrane metallopeptidases. J. Biol. Chem., 288(29):
21279-21294.
Summary for family M48. (https://merops.sanger.ac.uk/cgi-bin/famsum?family=m48).
