Custom MemPro™ Mitochondrial and Plastid Porins (MPPs)
Creative Biostructure provides custom MemPro™ gene-to-structure services for MPPs.
The mitochondrial and plastid porin (MPP) Family belongs to porin superfamily I. these members of porin superfamily I possess a large range of transmembrane β-strands (β-TMS), which are linked together by β-turn on the cytoplasmic side and long loops of amino acids on the other. Notably, the porin proteins translocate across mitochondrial and plastid outer membranes and act as a pore through which small molecules can diffuse. MPPs are a group of voltage-dependent anion-selective channel membrane proteins (VDACs) sharing highly conserved structural and electrophysiological characteristics in all eukaryotes, which are expressed in both mitochondrial outer membranes and plasma membranes. VDACs play an essential role in forming the mitochondrial permeability transition pore complex (PTPC) that controls the influx of various small molecules, including Ca2+, glutamate, NADH and nucleotides. In addition, VDACs are involved in complex interactions regulating organellar and cellular metabolism. In mammalian, there are three VDAC isoforms, including VDAC1, VDAC2 and VDAC3. It has been shown that mouse VDAC isoforms have different channel properties, however, they all display a slightly anionic-selective permeability of a large range of ions in the “open” state and a slightly cationic-selective permeability in the “closed” state.
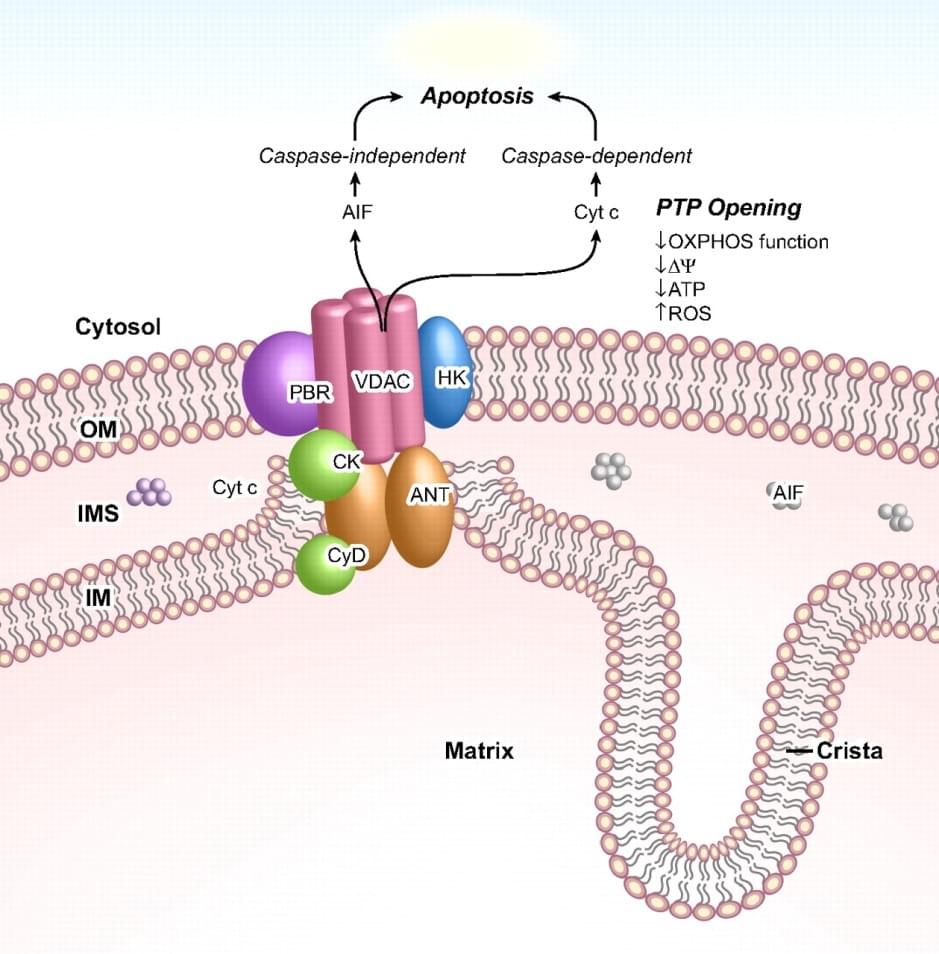
Figure 1. PTPC and its function in mediate apoptosis
VDAC1 is involved in the process of apoptosis by mediating the release of apoptosis-associated proteins (e.g., cytochrome C and AIF) from mitochondrion. Interestingly, this protein has been observed to be over-expressed in many cancer types. The characterization of VDAC disorder may cause a persistent opening of PTPC and result in the loss of membrane potential, ATP depletion and finally cell death. Erastin, a selective anti-tumor agent, can be used to bind VDAC2 and induce non-apoptotic cell-death in tumor cells. It is reported that RNA-interference (RNAi) knockdown mutant of VDAC2 or VDAC3 could cause resistance to erastin.
Creative Biostructure can provide various custom MemPro™ gene-to-structure services for membrane protein family, including the identification, isolation, purification, stabilization, and crystallization of membrane proteins of your interest. Please feel free to contact us for a detailed quote.
References:
E. J. Weeber, et al. (2002). The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J. Biol. Chem., 277(21): 18891-18897.
K. Fischer, et al. (1994). Porins from plants. Molecular cloning and functional characterization of two new members of the porin family. J. Biol. Chem., 269(41): 25754-25760.
K. Gabriel, et al. (2001). The alpha and the beta: protein translocation across mitochondrial and plastid outer membranes. Trends Biochem. Sci., 26(1): 36-40.
M. J. Young, et al. (2007). The evolutionary history of mitochondrial porins. BMC Evol, Biol., 7(31): 21.
Porin (protein). (https://en.wikipedia.org/wiki/Porin_%28protein%29)