Custom MemPro™ Ecto-nucleotide Pyrophosphatase/Phosphodiesterases (E-NPPs)
Creative Biostructure can provide unmatched custom MemPro™ gene-to-structure services for E-NPPs.
The E-NPP family is an important enzyme group mainly expressed in cartilage, bone and smooth muscle cells, and participates in nucleotide catabolism. E-NPPs rely on two Zn2+ in the catalytic core to hydrolyze phosphodiester bond in nucleotides and other substrates.
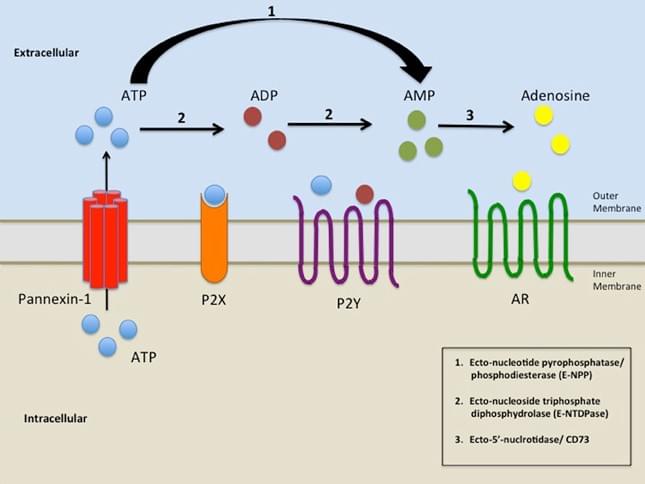 Figure 1. NPP role in Nucleotide catabolism. (Biomed. J., 2014)
Figure 1. NPP role in Nucleotide catabolism. (Biomed. J., 2014)
There are at least seven members in the NPP family, but only NPP1, NPP2 and NPP3 have been shown to hydrolyse nucleotides since they possess the nuclease-like domain and two somatomedin B-like domains to the plasma membrane. The three NPP proteins (NPP1-3) belong to type II transmembrane metalloenzymes characterized by a similar modular structure consisting of a short intracellular domain, a single transmembrane domain and an extracellular domain containing a conserved catalytic site. NPP1 has a longer intracellular domain than NPP2 and NPP3. NPP4 is mainly expressed on the surface of vascular endothelia, and regulates platelet aggregation by releasing ADP from Ap3A and activating the ADP receptors. Notably, NPP4 and NPP5 have a type I transmembrane orientation with a short intracellular carboxy-terminal domain. And so far, the function of NPP5 has been unknown. The short intracellular N-terminal (NPP1-3) and C-terminal (NPP4-5) domains show a striking lack of conservation between the different isoforms. NPP6 and NPP7 is believed to hydrolyze choline phosphate esters. It has been shown that NPP1-5 contain 4 to 10 putative N-glycosylation sites. These sites can contribute to transport E-NPPs correctly to the plasma membrane.
NPP shares great similarities with alkaline phosphatase (AP). However, AP primarily hydrolyzes monoester bonds; by contrast, NPP preferentially hydrolyzes phosphate diester bonds. NPP can also prevent the calcification of arteries and other soft issues. Thus, NPP deficiency promotes the spontaneous emergence of chondrogenesis in bone marrow stromal cells under non-calcifying conditions. NPP1-3 have been detected in almost all tissues, although individual isoforms are usually confined to specific substructures and/or cell types. In addition, NPP members have been localized in different cellular compartments and are differentially targeting to plasma membranes of polarized cells, demonstrating specific physiological functions of NPPs in cells and tissues.
Creative Biostructure can provide various custom MemPro™ gene-to-structure services for membrane protein family, including the identification, isolation, purification, stabilization, and crystallization of membrane proteins of your interest. Please feel free to contact us for a detailed quote.
References:
A. C. Morandini, et al.(2014). The role of p2x7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed. J., 37(4): 169-177.
Ectonucleotide pyrophosphatase/phosphodiesterase 1. (https://en.wikipedia.org/wiki/Ectonucleotide_pyrophosphatase/phosphodiesterase_1)
J. W. Goding, et al. (2003). Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochimica. et Biophysica. Acta (BBA), 1638(1): 1-19.
