Exosomes Isolated from Body Fluids
Exosomes isolated from bodily fluids, such as plasma, urine, and cerebrospinal fluid (CSF), are gaining increasing importance as tools for biomarker discovery and mechanistic disease studies. At Creative Biostructure, we specialize in providing high-quality, standardized exosome products derived from human and animal bodily fluids. Sourced from healthy donors, our exosomes are suitable for a variety of downstream applications, including enzyme-linked immunosorbent assays (ELISA), fluorescence-activated cell sorting (FACS), and Western blotting (WB).
Product List
Background
Body fluid-derived exosomes (BF-Exos) are a diverse group of extracellular vesicles secreted by various cell types found in fluids like blood, urine, saliva, CSF, and more. These exosomes are released by source cells into bodily fluids and are critical in intercellular communication, transferring information and regulating signals between cells and tissues.
Each type of BF-Exos offers unique biological insights. Plasma-derived exosomes are often preferred in research due to their preservation of exosomal integrity, avoiding interference from platelet-derived particles. Urine-derived exosomes, primarily from cells in the urinary tract, are useful for diagnosing and monitoring urinary system diseases. Saliva-derived exosomes provide information on oral and gastrointestinal health, while CSF-derived exosomes are invaluable for investigating neurodegenerative disorders. Additionally, exosomes from amniotic fluid offer potential in fetal development and maternal health studies, and breast milk-derived exosomes, particularly from mammalian sources like cow's milk, have garnered attention for their role in drug delivery and skin health research.
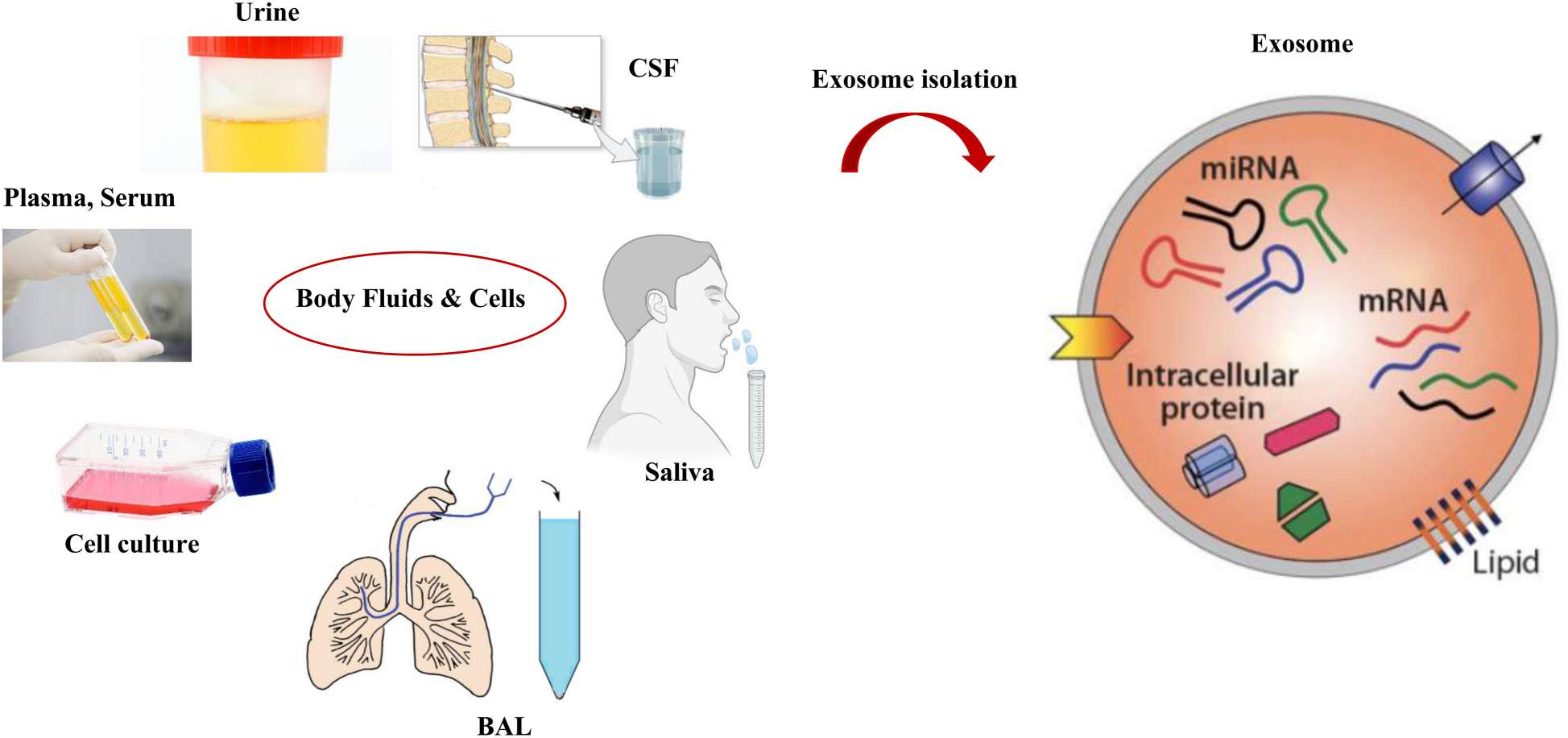 Figure 1. Overview of exosome isolation from various body fluids. (Jafari A, et al., 2023)
Figure 1. Overview of exosome isolation from various body fluids. (Jafari A, et al., 2023)
Applications of Body Fluid Derived Exosomes
Biomarker Discovery
Since exosomes carry molecular cargo reflective of their cell of origin, they can serve as miniature "snapshots" of the physiological or pathological state of tissues. This makes them ideal for identifying disease-specific proteins or RNA that can act as biomarkers, offering a non-invasive means to diagnose diseases at an early stage.
In cancer research, for example, tumor-derived exosomes often carry oncogenic proteins or mutated RNA that are specific to certain types of cancer. Detecting these molecules in bodily fluids, such as blood plasma or urine, allows for early diagnosis and monitoring of cancer progression without the need for invasive biopsies. Similarly, in neurodegenerative diseases like Alzheimer's or Parkinson's, exosomes isolated from CSF may contain pathological proteins such as amyloid-beta or tau, providing early biomarkers of disease that could revolutionize how we diagnose and monitor these conditions.
Mechanistic Studies in Disease Progression
Exosomes are not only useful for identifying biomarkers but also for understanding the mechanisms of disease. Their cargo reflects the physiological state of the donor, offering researchers a snapshot of cellular processes at any given time. For instance, exosomes isolated from cancer-state body fluids can be used to study how cancer cells communicate with their microenvironment, promoting tumor growth and metastasis. Similarly, exosomes derived from a single donor with a neurodegenerative disease may carry pathological proteins, such as amyloid-beta or tau, offering a non-invasive way to study disease progression and test potential therapeutics.
Drug Delivery
Exosomes are promising drug delivery vehicles due to their ability to penetrate barriers like the blood-brain barrier, their biocompatibility reducing immune responses, and their capacity to carry therapeutic agents directly to target cells, minimizing side effects. For example, milk-derived exosomes are particularly promising for drug delivery due to their abundance, ease of isolation, and natural stability, making them suitable for oral delivery. Their phospholipid bilayer structure, akin to cell membranes, protects therapeutic molecules from degradation in the stomach and intestine. Milk exosomes efficiently cross biological barriers, target specific tissues, and offer excellent biocompatibility, low immunogenicity, and high stability, making them an ideal platform for targeted drug delivery and a valuable tool in exosome research.
Case Studies
Case Study 1: Stability and Isolation of Plasma-Derived Exosomes
This study assessed the stability and optimized isolation techniques of exosomes derived from normal human blood plasma. It compared three methods: differential centrifugation, EpCAM-based immunoaffinity pull-down, and density gradient separation. Furthermore, the stability of plasma exosomes was confirmed over 90 days under various storage conditions, reinforcing their potential use in biomarker discovery and drug delivery applications.
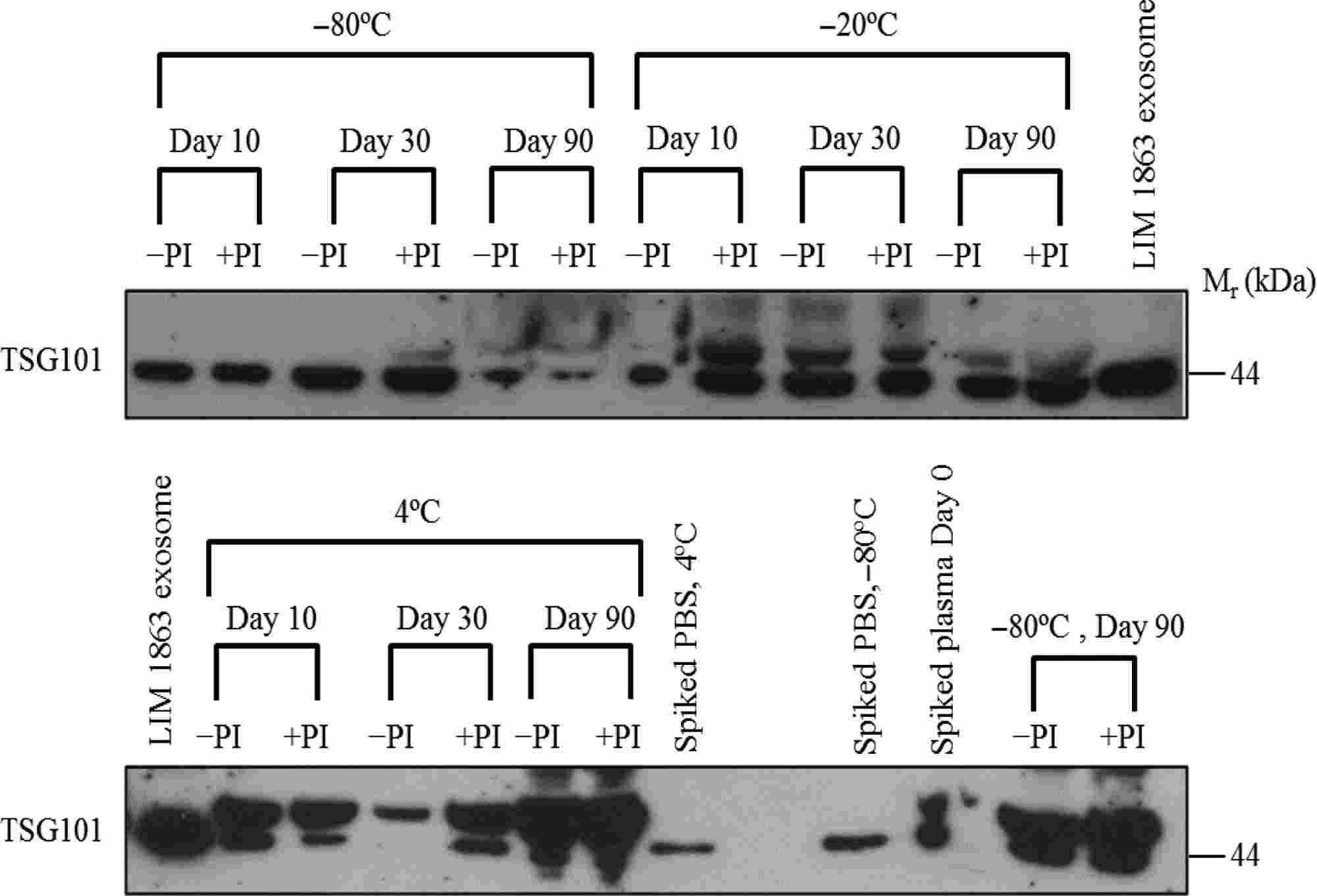 Figure 2. Assessment of plasma exosome stability at various storage temperatures. Western blot analysis of plasma samples spiked with
LIM1863 exosomes and stored at different temperatures (4°C, -20°C, and -80°C) over 10, 30, and 90 days. Exosomal stability was assessed by
detecting the TSG101 protein, indicating that exosomes remain stable at all tested conditions, with the highest stability observed at -80°C. (Kalra H,
et al., 2013)
Figure 2. Assessment of plasma exosome stability at various storage temperatures. Western blot analysis of plasma samples spiked with
LIM1863 exosomes and stored at different temperatures (4°C, -20°C, and -80°C) over 10, 30, and 90 days. Exosomal stability was assessed by
detecting the TSG101 protein, indicating that exosomes remain stable at all tested conditions, with the highest stability observed at -80°C. (Kalra H,
et al., 2013)
Case Study 2: Breast Milk-Derived Exosomes and Their Role in Breast Cancer Progression
This study explores exosomes derived from the breast milk of healthy lactating women and their influence on breast cancer. The research demonstrates that breast milk exosomes, particularly those containing high levels of TGFβ2, can induce epithelial-mesenchymal transition (EMT) in both benign and malignant breast epithelial cells. These exosomes significantly alter cell morphology and protein expression, promoting a more aggressive cancer phenotype.
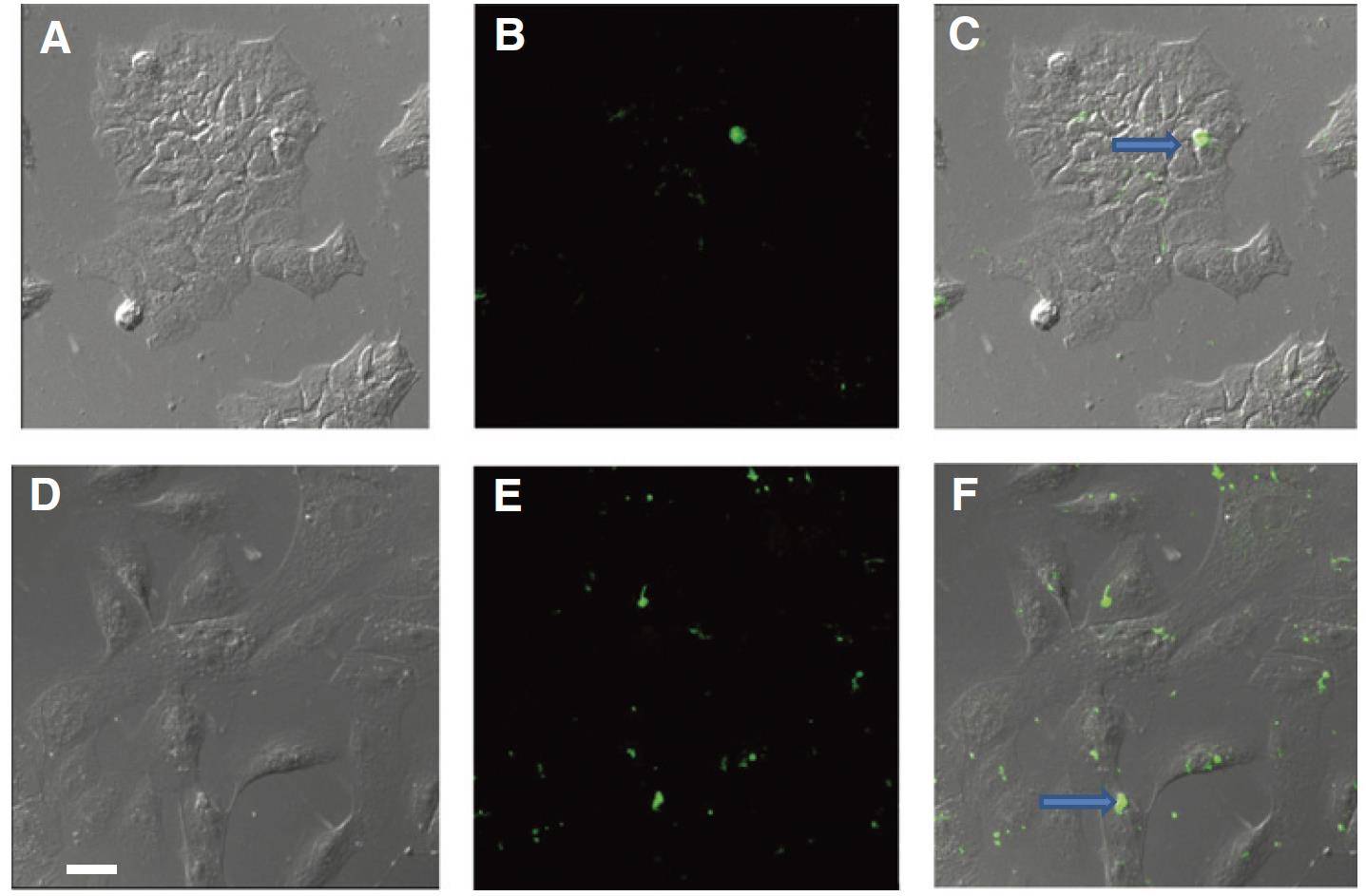 Figure 3. Confocal imaging of breast cells treated with breast milk-derived exosomes. Confocal images of MCF7 (A-C) and MCF10A (D-F)
cells cocultured with breast milk-derived exosomes. The images show GFP-labeled exosomes binding to CD63, a common exosome marker. (A and D) Without GFP; (B
and E) GFP with CD63 staining; (C and F) Merged images showing internalized exosomes (indicated by arrows). Scale bar: 25 µm. (Qin W, et al.,
2016)
Figure 3. Confocal imaging of breast cells treated with breast milk-derived exosomes. Confocal images of MCF7 (A-C) and MCF10A (D-F)
cells cocultured with breast milk-derived exosomes. The images show GFP-labeled exosomes binding to CD63, a common exosome marker. (A and D) Without GFP; (B
and E) GFP with CD63 staining; (C and F) Merged images showing internalized exosomes (indicated by arrows). Scale bar: 25 µm. (Qin W, et al.,
2016)
Product Advantages
- Diverse Body Fluid Sources: We provide exosomes isolated from various human and animal fluids, including human CSF, plasma, serum, urine, saliva, pleural, nasal fluid, sweat, tears, and animal plasma and milk.
- High Purity and Yield: Using advanced techniques such as ultracentrifugation, density gradient separation, and immunoaffinity capture, our optimized isolation process removes unwanted components (e.g., large extracellular vesicles and high-abundance plasma proteins) while preserving exosome integrity. This ensures minimal contamination, making them suitable for sensitive applications like WB, ELISA, and mass spectrometry.
- Stringent Quality Control: Each exosome batch undergoes rigorous quality control, including nanoparticle tracking analysis (NTA) for particle size and concentration, Western blotting for exosomal markers (CD63, CD81, TSG101), and transmission electron microscopy (TEM) for structural integrity assessment.
- Customizable Products: Beyond our standard offerings, we provide custom exosome isolation services tailored to specific research needs, accommodating preferences for donor populations, fluid types, or disease states. Our custom services also extend to modifications such as labeling exosomes with fluorescent markers for in vivo tracking or modifying their surface proteins for targeted delivery studies.
Resources
Frequently Asked Questions
-
What is the main advantage of using exosomes from bodily fluids over whole fluid samples?
Exosomes offer several advantages over whole fluid samples, primarily due to their ability to reduce sample complexity. Exosomes encapsulate disease-specific proteins, RNA, and other molecules, providing a more targeted and less complex sample for biomarker discovery and mechanistic studies. This is particularly useful in bodily fluids such as plasma, where high-abundance proteins can obscure low-abundance disease markers.
-
Can your exosomes be used for therapeutic purposes?
Our exosome products are designed for research use only and are not intended for therapeutic or clinical applications. However, they are suitable for investigating potential therapeutic uses of exosomes in preclinical studies, such as drug delivery or regenerative medicine.
-
Are the exosomes derived from healthy donors?
Yes, most of the exosomes we offer are derived from healthy donors. However, we also provide exosomes sourced from single donors in various disease states, enabling researchers to study specific pathological conditions.
Ready to advance your research with high-quality, body fluid-derived exosomes? At Creative Biostructure, we offer a wide range of exosome products tailored to your specific needs, whether for biomarker discovery, disease mechanism studies, or drug delivery research. Contact us today to discuss how our exosome solutions can drive your project forward!
References
- Kalra H, Adda C G, Liem M, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013. 13(22): 3354-3364.
- Qin W, Tsukasaki Y, Dasgupta S, et al. Exosomes in human breast milk promote EMT. Clinical Cancer Research. 2016. 22(17): 4517-4524.
- Jafari A, Karimabadi K, Rahimi A, et al. The emerging role of exosomal miRNAs as biomarkers for early cancer detection: A comprehensive literature review. Technology in Cancer Research & Treatment. 2023. 22: 15330338231205999.



