Mempro™ Cell-Based Glycophorin Production
Creative Biostructure has a well-established membrane protein production platform through years of experience. We can provide customized Mempro™ transmembrane protein glycophorin production services by our cell-based expression system.
Our most applied and advanced method for membrane proteins production is Mempro™ cell-based protein production system. Glycophorin family is a type of membrane sialoglycoproteins of red blood cell. This membrane-spanning protein family includes four members: Glycophorin A, Glycophorin B, Glycophorin C and Glycophorin E. Glycophorins are heavily glycosylated and carry lots of sugar molecules. Glycophorin family is rich in sialic acid, giving a very hydrophilic-charged coat to the red blood cells. As a result, glycophorins are able to circulate without adhering to vessel walls or other cells. In this family, glycophorin A is the most well known member for its dimerization. There is a seven-residue-motif (LIxxGVxxGVxxT) which define the dimerization interface of GpA, and this model motif highly occurs in other transmembrane sequences to mediate helix-helix interaction between small residues.
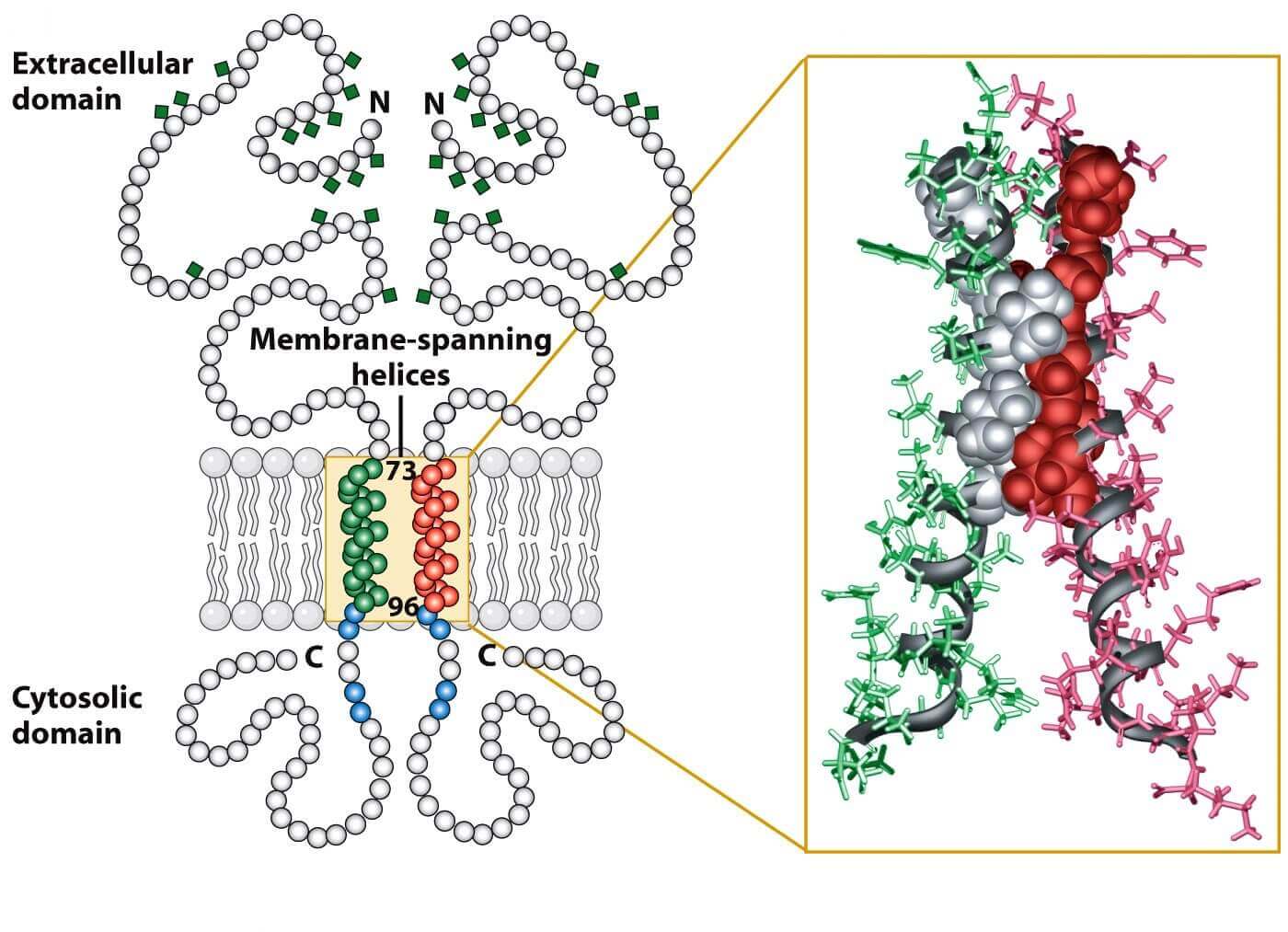 Figure 1. Structural basis for Glycophorin A dimerization.
Figure 1. Structural basis for Glycophorin A dimerization.
Creative Biostructure can provide you the best membrane protein production services, and we can apply a wide range of Mempro™ cell-based protein production systems including:
• Mempro™ Glycophorin Production in Bacterial Cells System
Escherichia coli (E. coli) is the most widely applied bacterial host for membrane protein production. Lemo21 (DE3) strain is optimized for our Mempro™ protein production in our bacterial cells system.
• Mempro™ Glycophorin Production in Insect Cells System
The baculoviruses are widely used for eukaryotic membrane protein production in cultured insect cells and insect larvae. According to similar codon usage rules, Mempro™ protein production using insect cells system can increase the expression level and reduce the truncated proteins compared to E. coli system. Due to similar codon usage rules, Mempro™ protein production using insect cells system can increase the expression level and reduce the truncated proteins compared to E. coli system.
• Mempro™ Glycophorin Production in Yeast Cells System
Single-celled yeast is an easy, quick and economic culture host and able to apply multiple post-translational modifications for eukaryotic membrane protein. Using this popular membrane production system, Creative Biostructure can provide Mempro™ cell-based Glycophorin production in yeast cells system.
• Mempro™ Glycophorin Production in Mammalian Cells System
Creative Biostructure can provide another great service-Mempro™ protein production in mammalian cells system. This system helps to correct folding and post-translational modifications for membrane protein. We have proprietary Mempro™ protein production in mammalian cells system with high quality eukaryotic membrane proteins from numerous mammalian cell types including human embryonic kidney cells (HEK-293), Chinese hamster ovary cells (CHO), monkey kidney fibroblast cells (COS-7) and baby hamster kidney cells (BHK-21).
Creative Biostructure provides Mempro™ cell-based protein production services from expressing, isolating, purifying to crystallizing the membrane protein Glycophorin, accelerating its structural and functional studies.
We provide an array of Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
T. Raphael, et al. (2015). Crystal Structure of the Glycophorin A Transmembrane Dimer in Lipidic Cubic Phase. J. Am. Chem. Soc., 137(50): 15676-15679.
K. Joseph, et al. (2016). Ligation of Glycophorin A Generates Reactive Oxygen Species Leading to Decreased Red Blood Cell Function. Plos One, 11(1): e0141206.
W. P. Russ, et al. (2000). The GxxxG Motif: A Framework for Transmembrane Helix-Helix Association. J. Mol. Biol., 296(3): 911-919.
B. Brosig, et al. (1998). The dimerization motif of the glycophorin A transmembrane segment in membranes: Importance of glycine residues. Protein Sci., 7(4): 1052-1056.