Exosomes Isolated from Immune-related Cell Lines
Exosomes isolated from immune-related cell lines offer significant potential in the study of immune regulation, cancer immunotherapy, and disease diagnostics. At Creative Biostructure, we isolate and purify exosomes from immune cells with high precision, ensuring that the exosomes retain their natural composition and functional integrity. These exosomes can be used as reference standards for exosome quantification in biological samples or as research tools in studying immune-related diseases.
Product List
Background
Immune system cell-derived exosomes (IEXs) are critical players in modulating immune responses and the progression of various diseases. These extracellular vesicles are secreted by immune cells such as T cells, B cells, dendritic cells, and macrophages. They carry a complex cargo of proteins, lipids, and nucleic acids, which allows them to influence immune cell activity, gene expression, and cytokine production. IEXs have been implicated in a wide range of immune-related diseases, including asthma, autoimmune disorders, cardiovascular diseases, and cancer. The ability of exosomes to either enhance or suppress immune responses underlines their dual role in disease progression and immune regulation.
Exosome biogenesis begins within late endosomes, where multivesicular bodies (MVBs) form and mature before fusing with the plasma membrane to release their exosome cargo into the extracellular space. Once released, exosomes can travel to target cells, delivering their molecular cargo and altering the target cells' function. In diseases such as cancer, exosomes secreted by tumor cells promote immune evasion by suppressing anti-tumor immunity, thereby facilitating tumor growth, angiogenesis, and metastasis. In contrast, immune cell-derived exosomes can stimulate immune responses, presenting antigens to T cells and enhancing antitumor immunity.
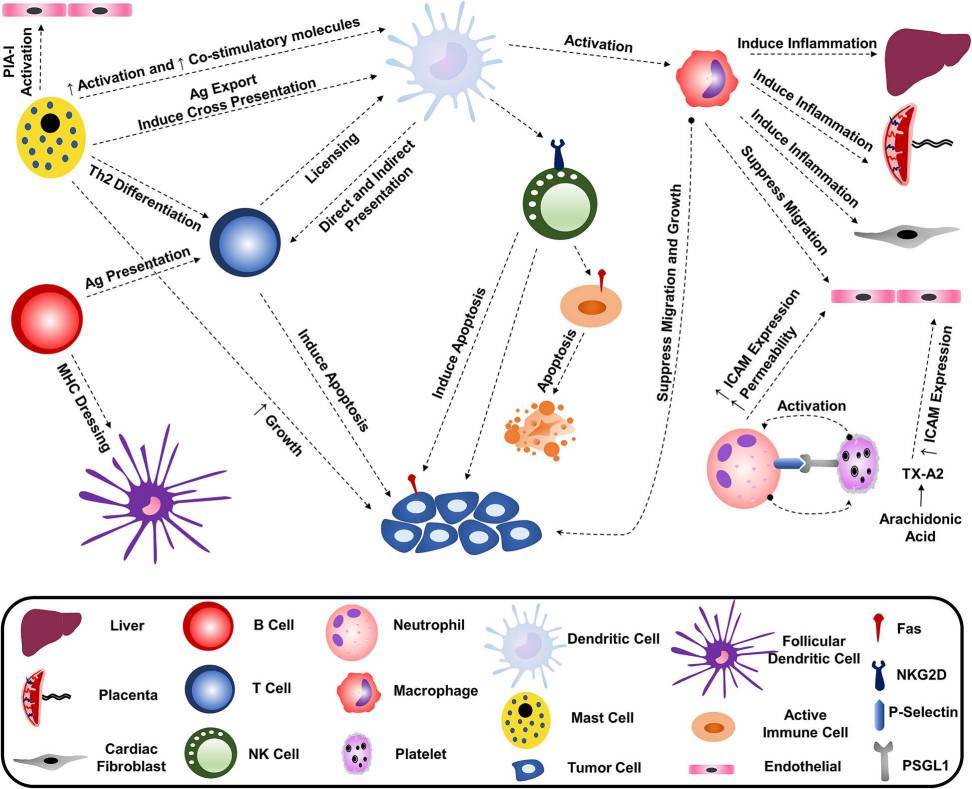 Figure 1. Immune cells communicate by secreting exosomes into their surroundings, influencing the function of other immune cells. (Hazrati A, et al., 2022)
Figure 1. Immune cells communicate by secreting exosomes into their surroundings, influencing the function of other immune cells. (Hazrati A, et al., 2022)
Applications of Immune System Cell-Derived Exosomes
Tumor Immunology: Modulating the Tumor Microenvironment
In cancer research, exosomes are key mediators of communication within the tumor microenvironment. Tumor-derived exosomes promote immune suppression, helping cancer cells evade detection and destruction by the immune system. They can inhibit cytotoxic T cells and natural killer cells while enhancing regulatory T cell activity, which dampens immune responses. By contrast, exosomes from immune cells like dendritic cells and macrophages can stimulate immune responses by presenting tumor antigens and activating T cells. These IEXs hold great promise for cancer immunotherapy, where manipulating exosomes could potentially reinvigorate anti-tumor immunity.
Immune Regulation: Modulating Inflammatory and Autoimmune Responses
Exosomes derived from immune cells are not only essential for activating immune responses but also for maintaining immune homeostasis. IEXs can carry molecules that modulate immune cell activity, either stimulating inflammation or promoting immune tolerance. For example, macrophage-derived exosomes can enhance inflammatory responses in diseases such as rheumatoid arthritis and multiple sclerosis, whereas exosomes from regulatory T cells can suppress excessive immune activation, preventing tissue damage in autoimmune diseases.
Targeted Drug Delivery: Exosomes as Natural Nanocarriers
One of the most promising applications of immune cell-derived exosomes is their potential use as drug delivery vehicles. Exosomes have the ability to naturally target specific tissues or cell types due to the proteins and receptors on their surface. This makes them ideal candidates for delivering therapeutic agents, such as small molecules, RNA, or proteins, directly to diseased cells while minimizing off-target effects.
Disease Diagnostics: Exosomal Biomarkers for Early Detection
Exosomes are also emerging as critical biomarkers for disease diagnosis. Because exosomes reflect the molecular content of their parent cells, their presence in body fluids such as blood, urine, and saliva can provide a non-invasive method for early disease detection. For example, exosomes from cancer cells carry tumor-specific markers that can be used to detect the presence of cancer before clinical symptoms appear. Similarly, exosomes derived from immune cells can indicate the activation or suppression of immune responses in diseases such as autoimmune disorders and infections.
Case Studies
Case Study 1: Dendritic Cell-Derived Exosomes in Antigen Presentation
This study uncovers a novel mechanism by which dendritic cells (DCs) enhance antigen presentation through the release of exosomes in conjunction with phagocytosed pathogens. The researchers demonstrate that the phagocytic uptake of Escherichia coli by DCs not only activates the cells but also triggers the release of small extracellular vesicles (EVs), which are enriched with major histocompatibility complex (MHC) molecules loaded with pathogen-derived peptides. This process suggests a strategic role for exosomes in selectively presenting antigens to the immune system, potentially improving vaccine design and understanding of immune responses.
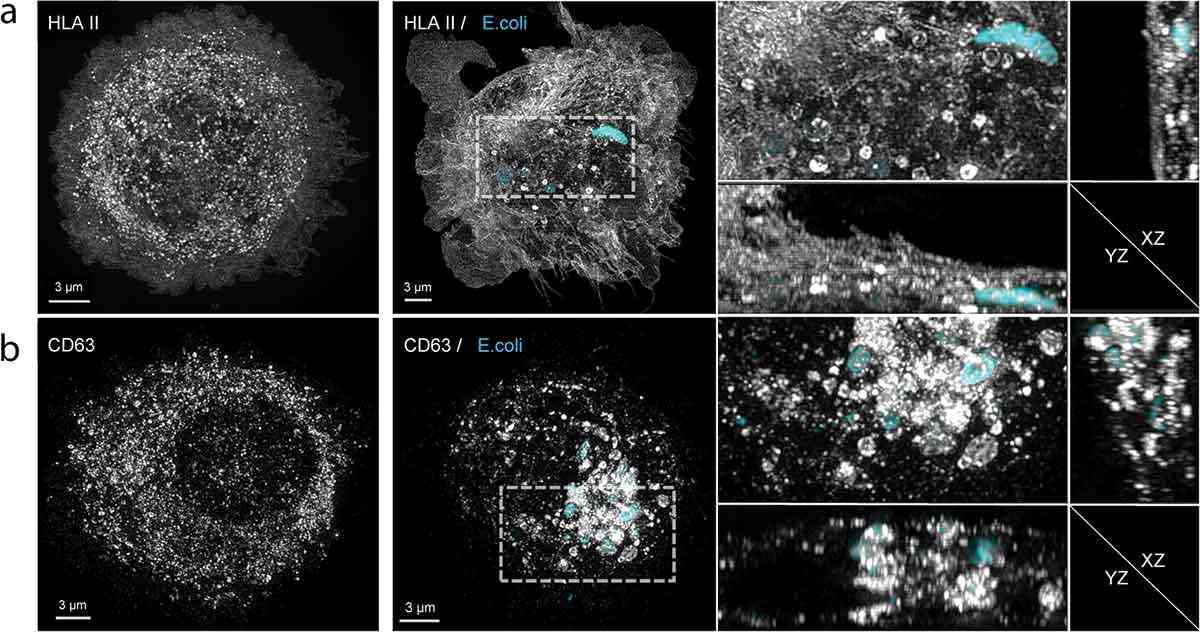 Figure 2. Structured illumination microscopy (SIM) images illustrating the relocation of HLA II and CD63 within dendritic cells (moDCs) following phagocytosis of E. coli. Panel (a) contrasts the distribution of HLA II in immature moDCs with that in activated moDCs after a 16-hour co-culture with Cy3B-labeled E. coli. There is a clear shift in HLA II localization from internal compartments to the cell membrane upon activation, with a notable concentration around the phagosomes containing E. coli. Panel (b) shows a similar analysis for CD63, an exosome marker, highlighting its accumulation in the vicinity of E. coli-engulfed phagosomes. (Lindenbergh M F S, et al., 2020)
Figure 2. Structured illumination microscopy (SIM) images illustrating the relocation of HLA II and CD63 within dendritic cells (moDCs) following phagocytosis of E. coli. Panel (a) contrasts the distribution of HLA II in immature moDCs with that in activated moDCs after a 16-hour co-culture with Cy3B-labeled E. coli. There is a clear shift in HLA II localization from internal compartments to the cell membrane upon activation, with a notable concentration around the phagosomes containing E. coli. Panel (b) shows a similar analysis for CD63, an exosome marker, highlighting its accumulation in the vicinity of E. coli-engulfed phagosomes. (Lindenbergh M F S, et al., 2020)
Case Study 2: Exosomal PD-L1 as a Modulator of CD8+ T Cell Exhaustion in Tumor Metastasis
This study uncovers a pivotal role for exosomal PD-L1 in facilitating tumor-specific CD8+ T cell exhaustion, which futher promotes metastasis. The research demonstrates that melanoma-derived exosomes, enriched with PD-L1, can directly interact with CD8+ T cells, leading to their functional impairment and exhaustion. Genetic knockdown of key exosome secretion enzymes or therapeutic blockade of PD-L1 significantly ameliorates this process, suggesting exosomal PD-L1 as a potential therapeutic target and biomarker for immune checkpoint blockade therapies in cancer metastasis.
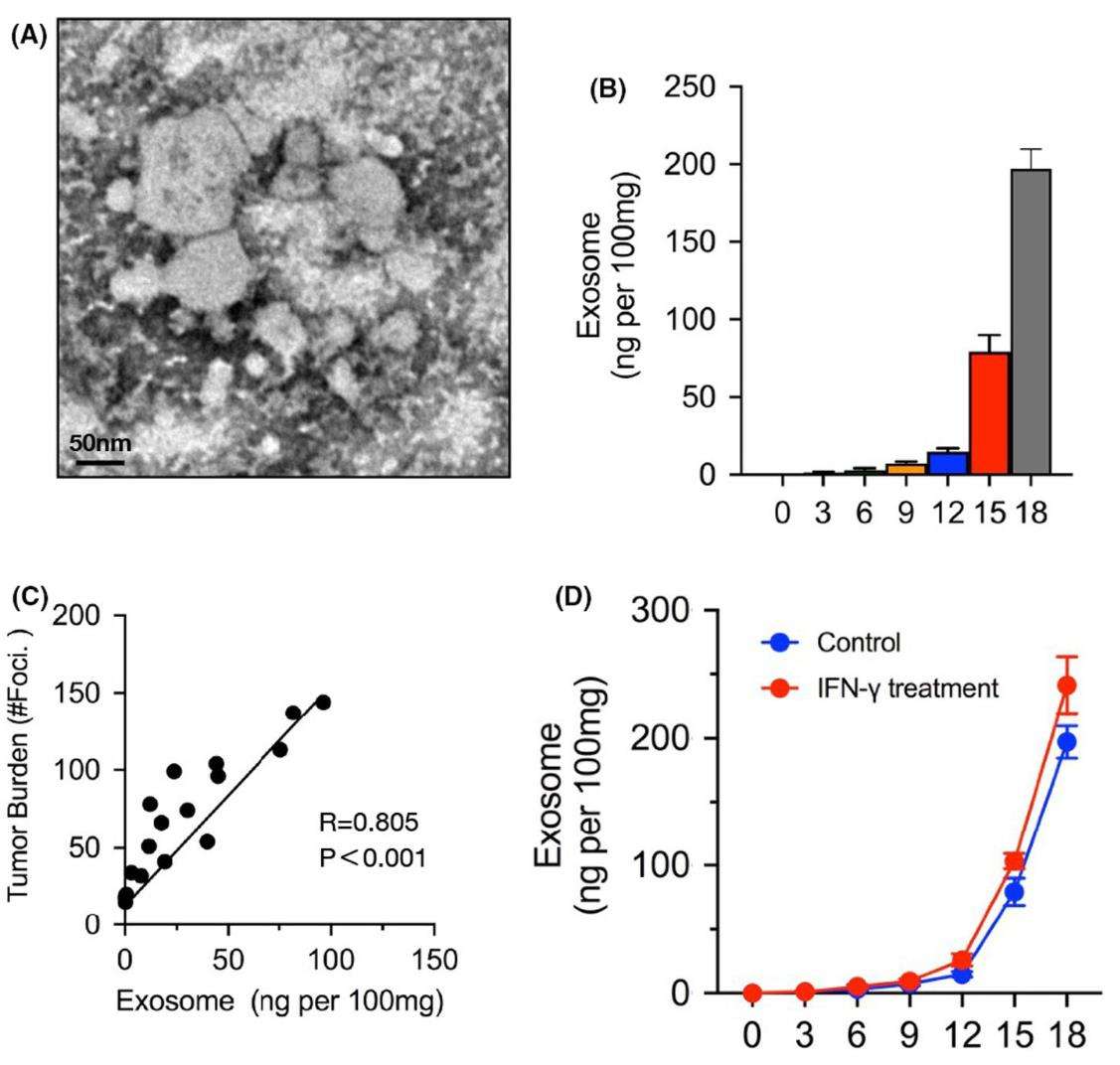 Figure 3. Elevated levels of melanoma-derived exosomes in metastatic progression. (A) Exosome morphology identified by electron microscopy in melanoma cell supernatants. (B) Exosome levels rise significantly during late-stage metastasis, as measured by ELISA. (C) A positive correlation exists between exosome levels and metastatic tumor burden. (D) IFN-γ treatment leads to a modest increase in exosome secretion, as detected by ELISA. (Chen J, et al., 2021)
Figure 3. Elevated levels of melanoma-derived exosomes in metastatic progression. (A) Exosome morphology identified by electron microscopy in melanoma cell supernatants. (B) Exosome levels rise significantly during late-stage metastasis, as measured by ELISA. (C) A positive correlation exists between exosome levels and metastatic tumor burden. (D) IFN-γ treatment leads to a modest increase in exosome secretion, as detected by ELISA. (Chen J, et al., 2021)
Why Choose Creative Biostructure?
- Superior Quality and Purity: We employ advanced techniques, including ultracentrifugation, immunoaffinity capture, and size exclusion chromatography, to isolate exosomes from immune-related cell lines with uncompromised structural and functional integrity, free from contaminants. This high purity makes them ideal for sensitive applications such as quantitative assays and functional studies.
- Customizable Solutions: Our exosome products are tailored to meet specific research needs, allowing for exosomes derived from particular immune cell types or designed for specific experimental conditions. This flexibility empowers researchers to execute detailed and confident experimental designs.
- Expert Support: Committed to client success, we provide comprehensive technical consultation, helping optimize experimental designs while offering post-delivery support to ensure complete satisfaction with our exosome products and services.
Resources
Frequently Asked Questions
-
What types of assays are your exosome products suitable for?
Our exosome standards are ideal for a wide range of research assays, such as Western blotting, ELISA, and flow cytometry.
-
How can exosome standards aid in diagnostic assay development?
Our exosome standards can be used as reference controls in diagnostic assays. They help quantify and identify exosomal biomarkers in body fluids, allowing researchers to develop reliable non-invasive diagnostic tools for conditions such as cancer, autoimmune diseases, and infectious diseases.
-
What quality control measures are in place for your exosome products?
Our exosome products undergo rigorous quality control, including size verification through nanoparticle tracking analysis (NTA), structural integrity checks with transmission electron microscopy (TEM), and purity assessments using flow cytometry. This ensures high reproducibility and consistency for research applications.
Explore our high-purity exosome products today and advance your immunological research with precision and confidence. Feel free to contact us for tailored solutions and explore our comprehensive product offerings.
References
- Lindenbergh M F S, Wubbolts R, Borg E G F, et al. Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation. Journal of Extracellular Vesicles. 2020. 9(1): 1798606.
- Chen J, Song Y, Miao F, et al. PDL1‐positive exosomes suppress antitumor immunity by inducing tumor‐specific CD8+ T cell exhaustion during metastasis. Cancer Science. 2021. 112(9): 3437-3454.
- Hazrati A, Soudi S, Malekpour K, et al. Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications. Biomarker Research. 2022. 10(1): 30.


