Nucleic Acid Vaccine Development Services for Coronavirus
Similar to recombinant virus vector vaccines, nucleic acid vaccines (including DNA vaccines and mRNA vaccines) can directly produce pathogen antigen proteins in the human body, which is an emerging vaccine technology. Creative Biostructure is accumulating preclinical research experience in nucleic acid vaccines, hoping to provide the optimal vaccine development services for different types of diseases caused by coronaviruses.
Brief Introduction to Nucleic Acid Vaccine
1. DNA vaccine
The DNA vaccine is to integrate the gene encoding the pathogen antigen protein into the plasmid, which is amplified in yeast or other cells, and then the recombinant DNA plasmids containing the antigen fragment are isolated and purified. The synthesis process of this type of vaccine is simple, and the stability of plasmid DNA at room temperature also increases its accessibility. However, due to the exogenous DNA, this kind of vaccine can induce anti-DNA antibodies and has the risk of insertion into the host cell genome. Another drawback is that the vaccines are less immunogenic, so adjuvants are needed to add for stimulating the body’s immune response.
2. mRNA vaccine
The advantage of the mRNA vaccine over the DNA vaccine is that it only needs to translate the target antigen in the cytoplasm, so there is no risk of integration into the host cell genome. However, the mRNA vaccine was initially ignored due to its poor stability and the lack of an effective in vivo delivery system. In recent years, with the development of technology, especially the development of polymer delivery systems, not only can the stability of mRNA be improved, but also its ability to enter cells can be increased. The mRNA can be synthesized in an in vitro reactor using DNA templates, nucleoside triphosphates, and RNA polymerase, and then purified and assembled into the delivery system to complete the production of the mRNA vaccine. According to this procedure, any given antigen can theoretically be produced in a short time, thus this type of vaccine is ideal for dealing with sudden outbreaks of infectious diseases. This is one reason why the mRNA vaccine against novel coronavirus is a standout in the vaccine development competition.
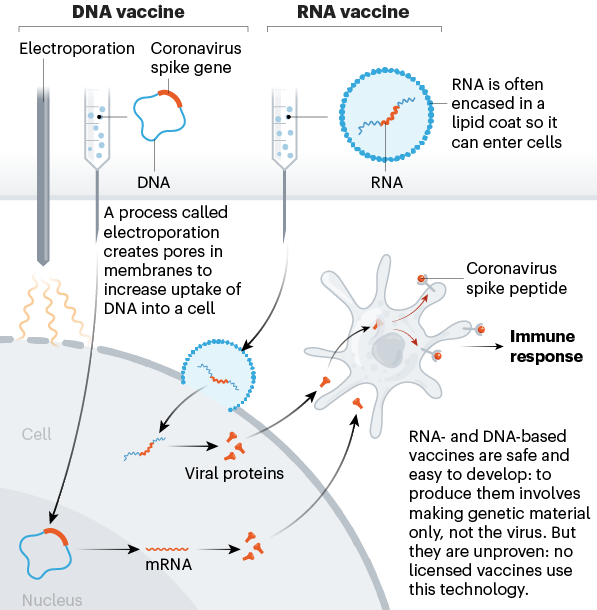 Figure 1. Nucleic-acid vaccines encoding the coronavirus spike protein. (Graphics: Nik Spencer/Nature)
Figure 1. Nucleic-acid vaccines encoding the coronavirus spike protein. (Graphics: Nik Spencer/Nature)
Nucleic Acid Vaccine Development Services for Coronavirus
Creative Biostructure currently provides the following services during the development of nucleic acid vaccines for coronavirus infection:
- Custom nucleic acid synthesis
We were able to customize and synthesize nucleic acid sequences with a wide array of modifications for multiple different antigens that are key targets of coronavirus at scales ranging from micrograms to milligrams. The mRNA can be generated from DNA templates provided by our customers or synthesized from scratch. The synthetic nucleic acid can be screened for applying in vaccine development.
- Liposome delivery technology solution
We are experts in liposome preparation and manufacturing. With the established liposome technology platform, we provide the optimal liposome services, from custom liposome production, characterization to application. We can support the packaging of nucleic acids into liposomes to ensure the safety and effectiveness of nucleic acid delivery. It has been proved that cationic liposomal formulations greatly promote the functional delivery of negative charged nucleic acids into cells.
We are committed to providing our customers with the most reliable and efficient research services to meet your research objectives. Want to know more? Please feel free to contact us. Our customer service representatives are available 24 hours a day from Monday to Sunday.
Contact us to discuss your project!
Reference
- Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020, 580(7805): 576.

 Figure 1. Nucleic-acid vaccines encoding the coronavirus spike protein. (Graphics: Nik Spencer/Nature)
Figure 1. Nucleic-acid vaccines encoding the coronavirus spike protein. (Graphics: Nik Spencer/Nature)