Surface Plasmon Resonance (SPR) for Coronavirus Research
Creative Biostructure is a leading service supplier in the field of structural biology with a team of specialists specializing in contract research services for basic and preclinical research related to coronavirus infection. Currently, we have established a surface plasmon resonance (SPR) method for protein interaction analysis associated with coronavirus research and support the development and optimization of SPR methods.
Brief Introduction to Surface Plasmon Resonance (SPR)
The global influence of Coronavirus Disease 2019 (COVID-19) makes the development of SARS-CoV-2 vaccines and antiviral drugs a priority for scientists around the world. The process of finding a solution involves understanding the biological molecular characteristics of the virus and the patterns of virus-host interaction through structural data, binding affinity, and kinetic data. SPR technology allows researchers to access binding affinity data in real time without using labels. It is an advanced technology in the field of biomolecular interactions. This technology can provide unique insight to reveal the interaction between proteins and other biological molecules and could help researchers to in-depth understand the functions of biomolecules, therefore facilitating the development of vaccines and drugs against coronavirus infection. For SPR analysis, the ligand of interest is immobilized on the surface of the biosensor chip, and the analyte is injected to flow over the surface of the chip. When the interaction between the ligand and the analyte occurs, the dynamic change of the SPR angle can be detected and analyzed for binding affinity.
Using SPR technology, the binding affinity between SARS-CoV-2 spike (S) protein and human angiotensin-converting enzyme 2 (ACE2) was analyzed. It is found that the binding affinity of human ACE2 protein to SARS-CoV-2 S protein is 10 to 20 times higher than that of SARS S protein.
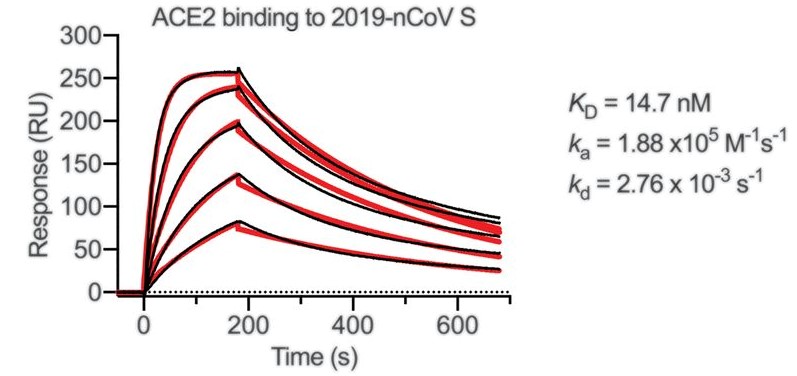 Figure 1. SPR sensorgram showing the binding kinetics for immobilized SARS-CoV-2 S protein and human ACE2. (Adapted from Wrapp D.; et al. 2020)
Figure 1. SPR sensorgram showing the binding kinetics for immobilized SARS-CoV-2 S protein and human ACE2. (Adapted from Wrapp D.; et al. 2020)
Our SPR Services for Coronavirus Research
In the past years, we have accumulated rich experience in SPR technology and many experimental data, such as bio-macromolecular interactions, antibody screening/characterization, as well as consistency evaluation of protein/antibody.
Our team of scientists with a shared scientific background in virology and biophysical technologies provides custom assay development services for coronavirus infection research and supports SPR experimental design and data analysis. We aim to help customers gain faster access to high-quality binding kinetics data so that you can better understand disease pathways, accelerate lead generation and candidate validation. At Creative Biostructure, we can help you:
- Detect binding affinity between molecules, allowing for protein-protein interaction (PPI) analysis and ligand binding dynamics analysis
- Screen antibodies, including preliminary affinity ranking of the candidate antibodies and analysis of the specificity and kinetic characteristics of the antibodies
- characterize antibodies, for further understanding the affinity and kinetic characteristics of antibodies and preliminarily judge the metabolism of antibodies in vivo
- Evaluate consistency of protein/antibody to guarantee quality
Creative Biostructure has established a variety of methods involving cutting-edge techniques for the detection of biomacromolecule interactions. Please do not hesitate to contact us for more information. Our customer service representatives are available 24 hours a day from Monday to Sunday.
Contact us to discuss your project!
Reference
- Wrapp D.; et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation.Science. 2020, 367(6483): 1260-1263.

 Figure 1. SPR sensorgram showing the binding kinetics for immobilized SARS-CoV-2 S protein and human ACE2. (Adapted from Wrapp D.; et al. 2020)
Figure 1. SPR sensorgram showing the binding kinetics for immobilized SARS-CoV-2 S protein and human ACE2. (Adapted from Wrapp D.; et al. 2020)