Vaccine Development Services for Coronavirus
Over the past five years, the world has experienced several outbreaks of virus, including Ebola virus, Zika virus, MERS-CoV and SARS-CoV-2 (novel coronavirus, formerly termed as 2019-nCoV). Faced with the potential threat of a pandemic, research institutions and companies in multiple countries and areas around the world have announced vaccine development plans. Creative Biostructure is a leading contract service vendor in the field of structural biology, and with the virus-like particle (VLP) platform we have established, we will support the development of coronavirus vaccine more quickly and accurately.
Brief Introduction to Virus Vaccine Development
There are only about 70 vaccines are utilized to prevent 37 human infectious diseases, and few vaccines have been successfully marketed. Among the vaccines approved for marketing from 1880 to 2016, the shortest vaccine development cycle takes 10 years, and some take more than hundred years. However, with the emergence and development of R&D technologies for new vaccines, the speed of market launch of vaccines has gradually accelerated. According to different mechanisms and methods, vaccines are divided into the following types: whole-inactivated vaccines, synthetic peptide vaccines, recombinant viral vector vaccines, nucleic acid vaccines, live attenuated vaccines, recombinant protein vaccines, recombinant bacterial vector vaccines, and virus-like particle vaccines.
Vaccines for novel coronavirus do not necessarily solve our immediate problems. The research and vaccine development experience of these viruses that cause outbreaks will give us confidence in the face of emerging infectious diseases in the future.
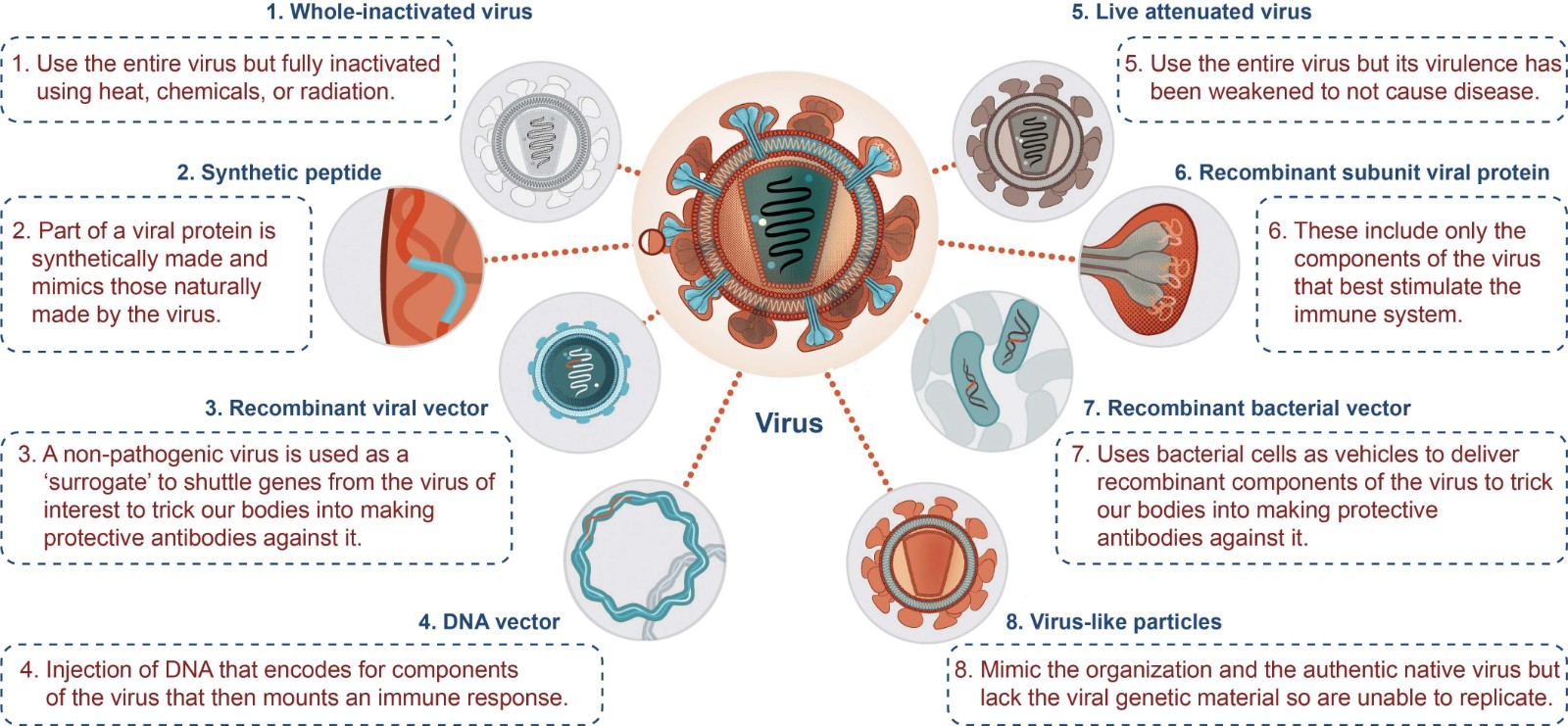 Figure 1. Types of virus vaccines. (Adapted from hvtn.org)
Figure 1. Types of virus vaccines. (Adapted from hvtn.org)
Vaccine Development Services at Creative Biostructure
Over the past decade, Creative Biostructure has established a state-of-the-art virus-like particle (VLP) platform and has provided satisfactory services for various VLP applications. Nowadays, we hope to utilize this platform to support the development of coronavirus vaccines. We are also working to gain experience in the discovery and development of novel structure-based vaccines. We are committed to providing efficient and cutting-edge technical support for the development of vaccines for coronavirus diseases and other emerging infectious diseases, including but not limited to the following types of vaccine development:
Why Do You Choose Us?
Creative Biostructure's expertise and experience in custom VLP design/development and structure-based antiviral vaccine research make us a reliable partner for your discovery of novel coronavirus vaccines. Vaccine development is a long and arduous process, and we would like to apply our insights on the target structure to the vaccine development process. We guarantee:
- Customized and flexible services
- Professional management and regular communication
- Customer privacy and data security
- Efficiency and cost-effectiveness
- Our customer service representatives are available 24 hours a day from Monday to Sunday
Contact us to discuss your project!

 Figure 1. Types of virus vaccines. (Adapted from hvtn.org)
Figure 1. Types of virus vaccines. (Adapted from hvtn.org)