The Middle East respiratory syndrome (MERS) epidemic caused by MERS-coronavirus (MERS-CoV) has a mortality rate of up to 35%, which is much higher than severe acute respiratory syndrome (SARS, mortality rate is about 10%), and compared with SARS, the disease progresses more rapidly. Since its discovery, most cases of MERS-CoV infection have mainly occurred in the Middle East. In 2015, the MERS epidemic in South Korea severely erupted, which attracted global attention. This is the second coronavirus with high pathogenicity to humans after SARS-CoV. At present, anti-MERS-CoV candidate drugs mainly include monoclonal antibodies, peptides, and small molecule compounds. A neutralizing human monoclonal antibody (m336) with high inhibitory activity against MERS-CoV has been successfully developed. This is one of the best therapeutic drugs targeting MERS-CoV at present, it has extremely strong virus-neutralizing activity, and the binding affinity constant with MERS-CoV reaches the picomolar level.
Taxonomy and Genome
MERS-CoV is an enveloped β-C coronavirus with a positive-stranded RNA genome. Its genome length is about 30.1 kb, and its genomic similarity to SARS-CoV is only 54.9%. The genome of MERS-CoV is composed of 30119 nucleotides, including more than 10 open reading frames (ORFs), encoding non-structural and structural proteins such as replicase, spike protein (S protein), envelope protein (E protein), membrane protein (M protein), nucleocapsid protein (N protein). Among them, the S protein has a binding site with the host cell receptor, which can mediate the virus to invade the host cell.
Structural Biology and Potential Drug Targets
Similar to other coronaviruses, MERS-CoV enters host cells mainly through endocytosis and membrane fusion. The S protein belongs to type-1 transmembrane protein, which is embedded in the viral envelope in the form of trimer. The S protein is synthesized in the cytoplasm of host cells and exists as a protein precursor. Only after being cleaved by the protease does it have the ability to mediate the virus entry into the host cell. Unlike the receptor of SARS-CoV (ACE2), the MERS-CoV receptor is dipeptidypeptidase4 (DPP4, also known as CD26). The S protein can be divided into S1 and S2. Among them, the receptor-binding domain (RBD) of S1 binds to the host DPP4 receptor on the cell membrane, and S2 changes its conformation accordingly, exposing the fusion peptide (FP) with membrane-penetrating function, which mediates the endocytosis or host cell membrane fusion with virus envelope.
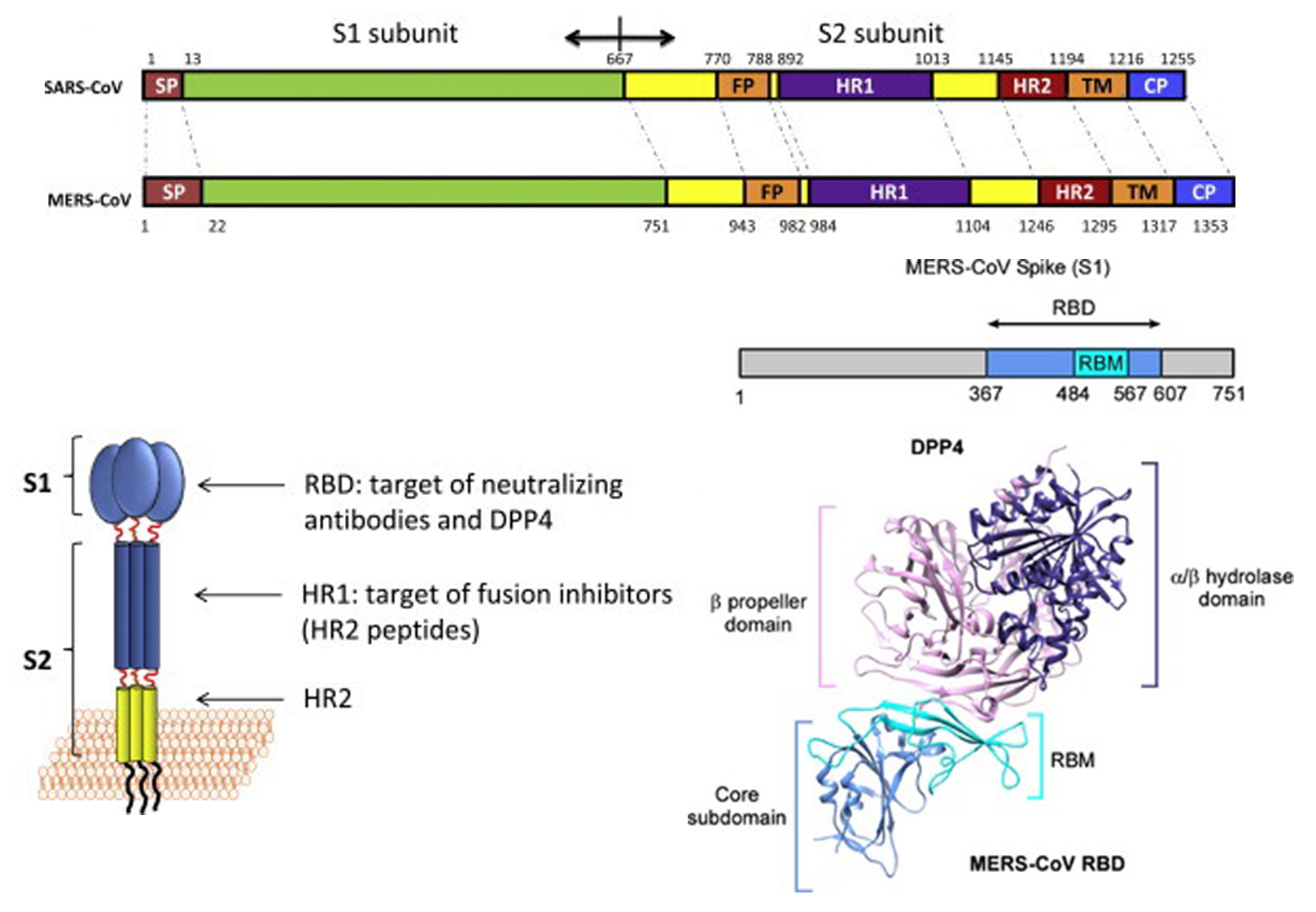 Figure 1. Structure of MERS-CoV S protein. (Adapted from Xia S.; et al. 2014 & Milne Price S.; et al. 2014)
Figure 1. Structure of MERS-CoV S protein. (Adapted from Xia S.; et al. 2014 & Milne Price S.; et al. 2014)
At the beginning of coronavirus replication, viral genes are directly translated into two protein precursors (pp1a, pp1ab), which are subsequently cleaved by viral proteolytic enzymes into non-structural proteins of the virus. Thus, viral proteolytic enzymes control the replication activity of the virus.
Proteins encoded by MERS-CoV itself and the host cell play significant roles in the whole process of virus infection and replication. Therefore, proteins involved in the viral infection and replication cycle (such as S protein, viral proteolytic enzyme, host cell protease, etc.) can be used as targets for anti-MERS-CoV drugs.
Contract Research Services for Coronavirus Infections
Creative Biostructure hopes to provide comprehensive basic research and preclinical research solutions for Middle East respiratory syndrome (MERS) and other coronavirus infections with its expertise in structural biology and years of experience. Below is a list of featured services:
 Figure 2. Our contract research services for coronavirus infections.
Figure 2. Our contract research services for coronavirus infections.
Contact us to discuss your project!
References
- Van Boheemen S.; et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012, 3(6).
- Qian Z, Dominguez S R, Holmes K V. Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation. PloS One. 2013, 8(10): e76469.
- Ying T.; et al. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. Journal of Virology. 2014, 88(14): 7796-7805.
- Xia S.; et al. Middle East respiratory syndrome coronavirus (MERS-CoV) entry inhibitors targeting spike protein. Virus Research. 2014, 194: 200-210.
- Milne Price S, Miazgowicz K L, Munster V J. The emergence of the Middle East respiratory syndrome coronavirus. Pathogens and Disease. 2014, 71(2): 121-136.

 Figure 1. Structure of MERS-CoV S protein. (Adapted from Xia S.; et al. 2014 & Milne Price S.; et al. 2014)
Figure 1. Structure of MERS-CoV S protein. (Adapted from Xia S.; et al. 2014 & Milne Price S.; et al. 2014) Figure 2. Our contract research services for coronavirus infections.
Figure 2. Our contract research services for coronavirus infections.