Protein Structure Analysis Using Single Particle Cryo-EM for Coronavirus Research
In recent years, with the advancement of electronic detectors, algorithms and other crucial factors, electron microscopy (EM) technology has been increasingly utilized to study the ultrastructure of macromolecules, cells and tissues. In particular, single-particle cryo-electron microscopy (Cryo-EM) has become a popular and effective method for studying the structure of biologically significant molecules (such as proteins and virus particles) at a near-atomic resolution to better understand the three-dimensional (3D) structure and function of biological macromolecules. Creative Biostructure, a professional contract service provider in the field of structural biology, hopes to apply years of expertise and experience in single-particle Cryo-EM technology to the structural analysis of coronavirus-related proteins and complexes to support the structure-based design of vaccines and antiviral drugs.
Brief Introduction to Coronavirus and SARS-CoV-2
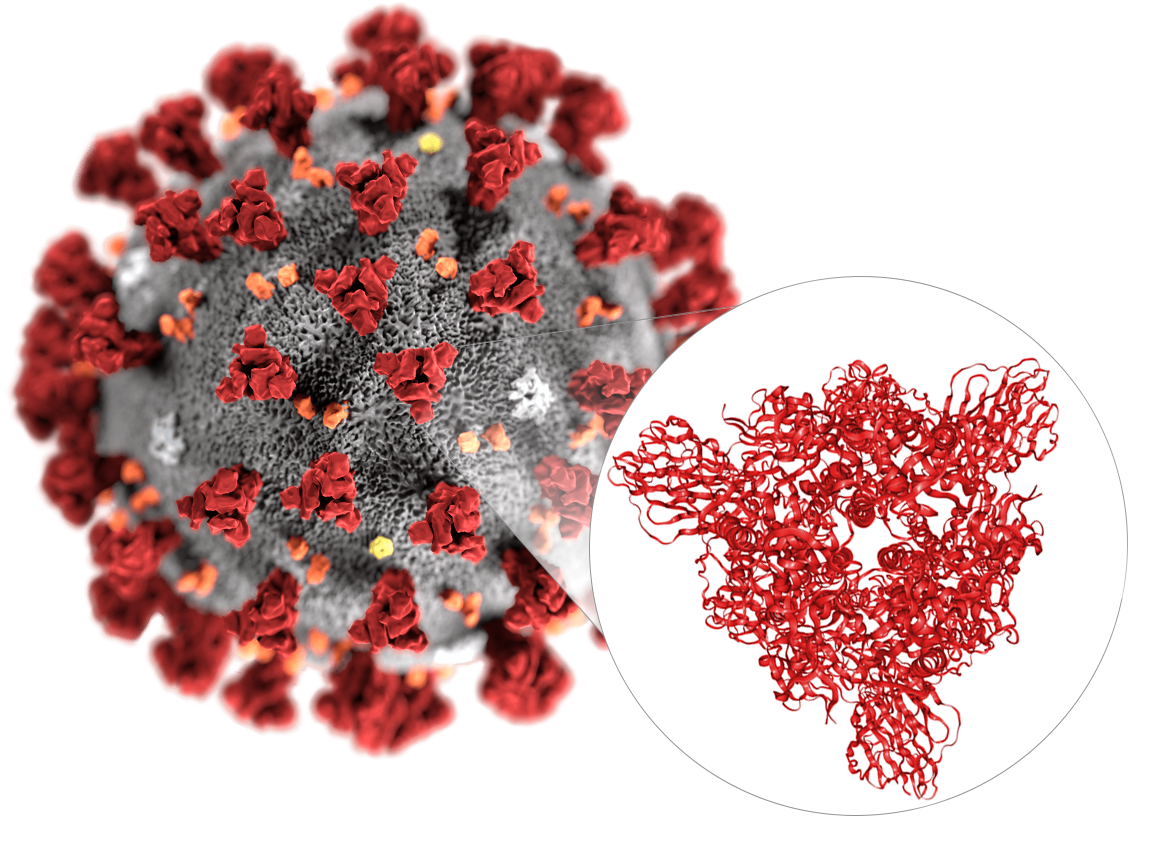
SARS-CoV-2 (formerly known as 2019-nCoV) is a novel virus that causes an outbreak of a respiratory disease known as COVID-19 and is the first coronavirus leading to a global pandemic. The invasion of coronaviruses mainly mediated by the binding of spike glycoprotein (S protein) with host cell receptors. The S protein is a large trimeric transmembrane glycoprotein. At present, scientists have successfully reconstructed the 3D structure of the S protein trimer on the surface of the SARS-CoV-2 using Cryo-EM and obtained the EM structure of the complex of the full-length cell receptor ACE2 protein and the SARS-CoV-2 receptor-binding domain (RBD). These findings provide an important structural biology foundation for further accurately vaccine design and antiviral drug discovery.
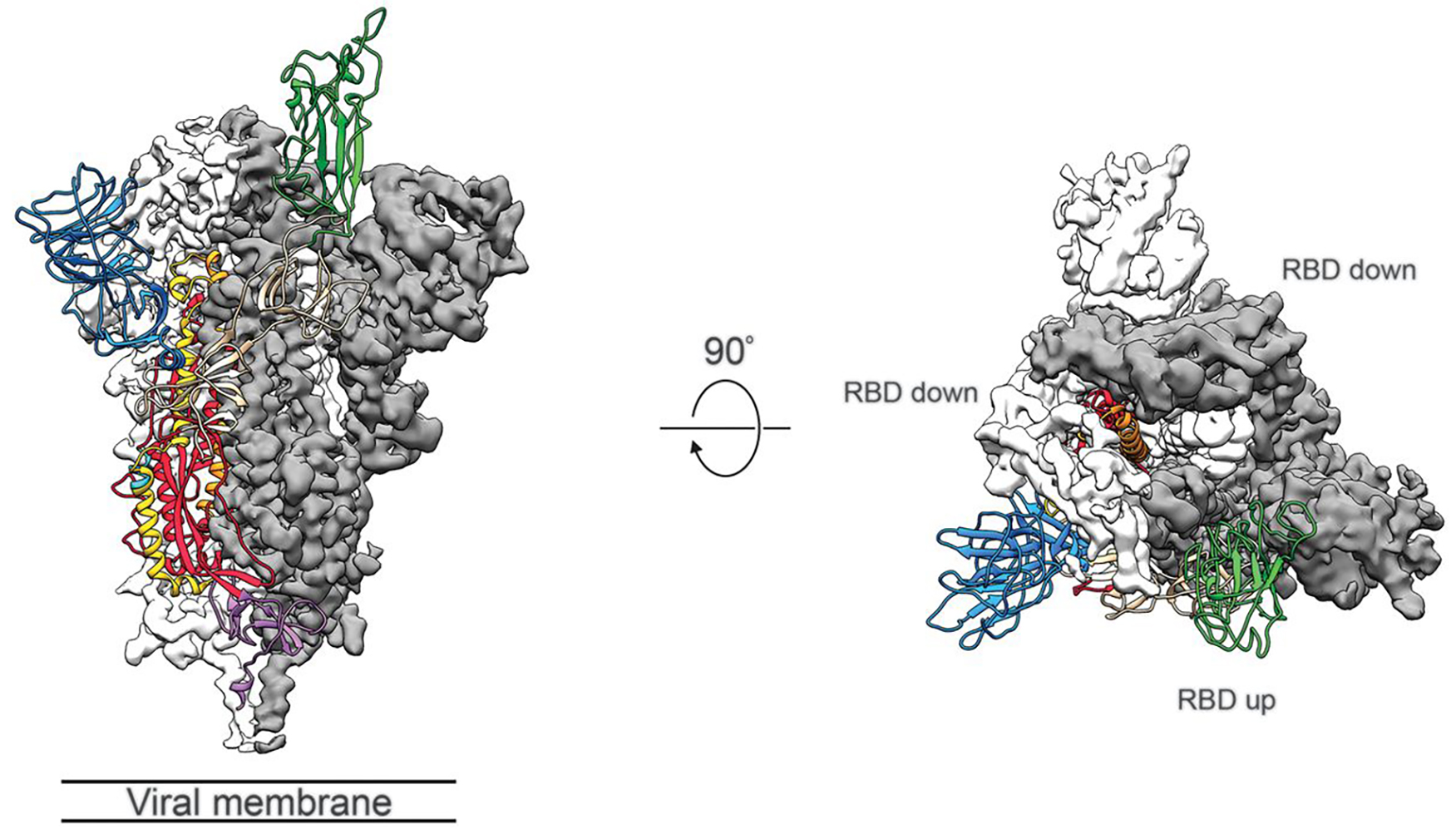 Figure 1. Structure of SARS-CoV-2 S protein in the prefusion conformation. (Adapted from Wrapp D.; et al. 2020)
Figure 1. Structure of SARS-CoV-2 S protein in the prefusion conformation. (Adapted from Wrapp D.; et al. 2020)
Why Do You Need This Service?
Cryo-EM single particle analysis (SPA) technology has made revolutionary advances, and its resolution is now even comparable to X-ray crystallography technology. Thus, this technology is becoming a popular tool for determining the high-resolution structure of biomacromolecules. Compared to X-ray crystallography technology, the rapid freezing of the samples keeps the protein in a near-natural state, and SPA allows simultaneous analysis of different conformations of the target protein. Moreover, this technology requires only a small amount of sample and is more tolerant to protein purity and does not require the protein to crystalize. If your research on coronavirus disease involves biological macromolecules or complexes that are difficult to crystallize, then our single-particle Cryo-EM analysis will efficiently provide you with structural details about these molecules.
What We Can Do?
Creative Biostructure can offer protein structure analysis involved in coronavirus research using single-particle Cryo-EM, supporting the development of structure-based vaccine and treatment development. In order to obtain the initial model or to quickly screen homogeneous samples, we recommend performing the negative staining EM experiment before observing the samples with Cryo-EM. Our services are comprehensive and unbundled, including but not limited to molecular cloning, protein expression, protein purification, Cryo-EM sample preparation, EM imaging, data processing, 3D model building and refinement.
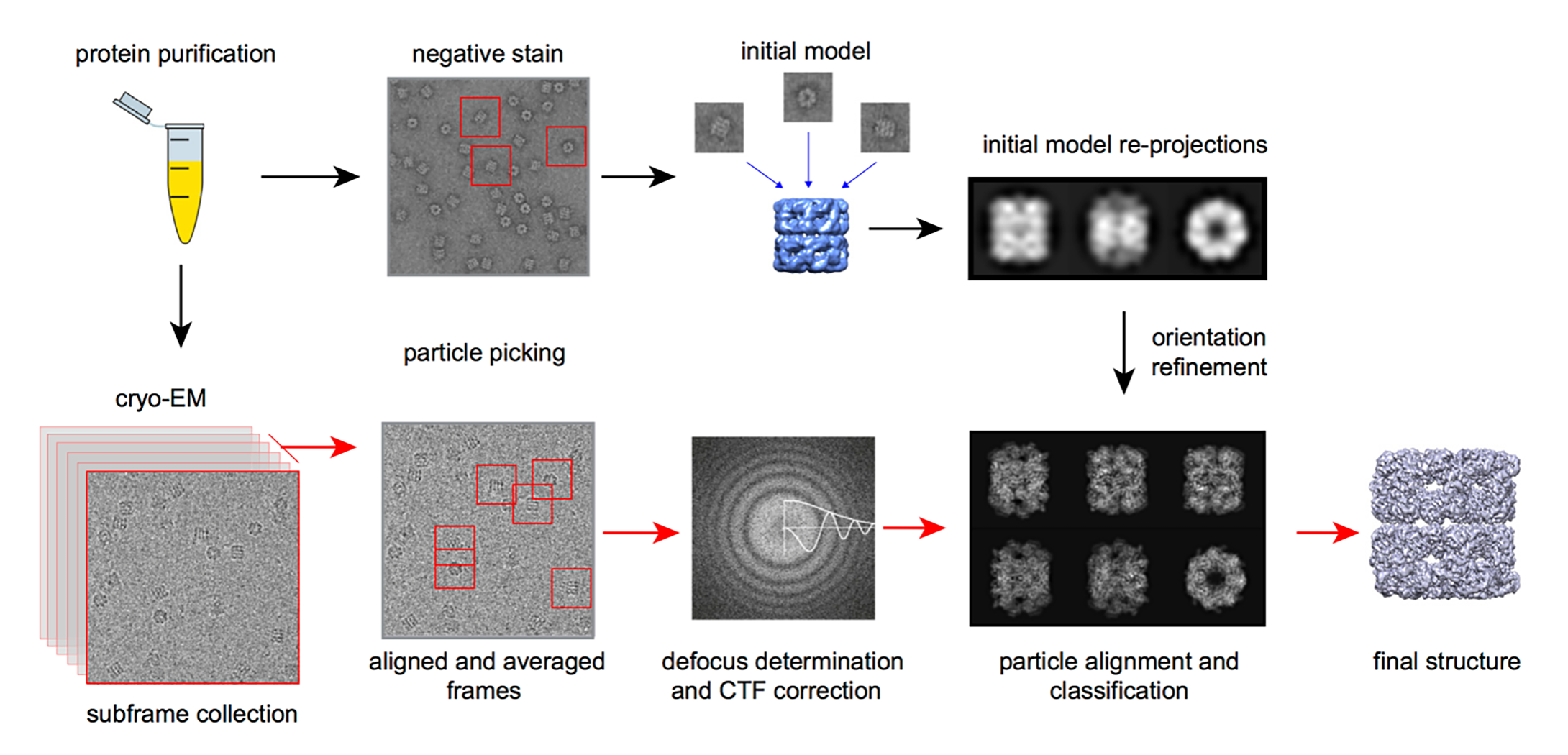 Figure 2. The workflow of single particle Cryo-EM technology.
Figure 2. The workflow of single particle Cryo-EM technology.
Why Do You Choose Us?
- Highly specialized instruments
- Broad expertise in sample preparing, EM imaging, and SPA data interpreting
- Quick response and efficient communication
- Flexible and high-quality service to fully meet customer needs
- Our customer service representatives are available 24 hours a day from Monday to Sunday
Contact us to discuss your project!
References
- Wrapp D.; et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020, 367(6483): 1260-1263.
- Yan R.; et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020, 367(6485): 1444-1448.
- Wang H W, Wang J W. How cryo-electron microscopy and X-ray crystallography complement each other. Protein Science. 2017, 26(1): 32-39.


 Figure 1. Structure of SARS-CoV-2 S protein in the prefusion conformation. (Adapted from Wrapp D.; et al. 2020)
Figure 1. Structure of SARS-CoV-2 S protein in the prefusion conformation. (Adapted from Wrapp D.; et al. 2020) Figure 2. The workflow of single particle Cryo-EM technology.
Figure 2. The workflow of single particle Cryo-EM technology.