Sun Fei research group of Institute of Biophysics, Chinese Academy of Sciences, and Hartmut Michel research group of Max Planck Research Institute, Germany published a research paper entitled “The unusual homodimer of a heme copper terminal oxide allow itself to utilize two electron donors” in German Applied Chemistry. In this work, a special heme copper terminal oxidase from thermophilic bacteria was studied.
By analyzing its high-resolution freeze electron microscope structure, they found its unique catalytic mechanism of oxidation of both cytochrome c and hydroquinone, and found that its proton channel and oxygen channel have novel structural characteristics. These results further enrich our understanding of the structural differences of cytochrome c oxidase among different species and reveal the evolutionary adaptability of cytochrome c oxidase in extreme environments.
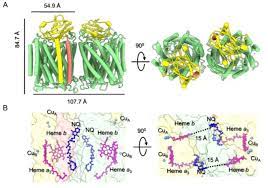
Heme copper terminal oxidase family proteins are a class of important metalloproteinases, which are responsible for the efficient transfer of electrons from cytochrome c or hydroquinone to oxygen molecules and the reduction of oxygen to water. The family members are multisubunit complexes, which are composed of 14 subunits in mammalian mitochondria and 3 subunits in most bacteria. Their core catalytic subunit I is very conservative and has two heme atoms and one copper atom, in which the copper atom forms a binuclear center with the iron atom of high spin heme; subunit II usually has two copper atoms to form a binuclear center. Cytochrome c oxidase is a member of the heme copper terminal oxidase family. Previous studies have shown that it can only use cytochrome c as an electron donor. There are significant differences in the structure of cytochrome c oxidase among different species, including the number of subunits, the number of transmembrane helix of subunit I / II, the structural characteristics of proton channel and oxygen channel, etc.
Aqui fecus is one of the oldest thermophilic bacteria in the world. In 2012, the Hartmut Michel team reported for the first time that this thermophilic bacterium has a unique cytochrome c oxidase, which can oxidize both cytochrome c and hydroquinone, but the catalytic mechanism and evolutionary significance are still unknown. After years of cooperation, Sun Fei and Hartmut Michel recently analyzed the three-dimensional structure of A. Aeolicus cytochrome c oxidase with a resolution of 3.4 angstroms by using the single-particle technique of frozen electron microscope.
It forms a unique homodimer, and its binding form is different from that of cytochrome c oxidase reported previously in other species. At the dimer interface, there are two substrate hydroquinone molecules (NQ) tightly bound on the complex, forming a potential electron transfer chain, which makes the whole cytochrome c oxidase oxidize both cytochrome c and hydroquinone. The study shows that this unique dimerization characteristic is necessary for double substrate catalysis. The structural analysis confirmed that the proton channel efficiency of A. Aeolicus cytochrome c oxidase decreased significantly, while the oxygen channel efficiency increased in order to adapt to hypoxia and high-temperature environment. In addition, similar to a. Aeolicus respiratory chain complex III, it also has some unique thermal stability structural characteristics. These results further enrich the understanding of the structure and function of cytochrome c oxidase. This is also another important development of the research team following the analysis of the structure of A. Aeolicus respiratory chain complex III, which is conducive to the in-depth understanding of the respiration of this ancient super thermophilic bacteria and the evolutionary strategy of the respiratory chain complex to adapt to extreme conditions.
Reference
Zhu Y, Zhu G, Zeng H, Zhang S, et al. The unusual homodimer of a heme-copper terminal oxidase allows itself to utilize two electron donors. Angew Chem Int Ed Engl. 2021 Mar 4. doi: 10.1002/anie.202016785.