To treat a disease, we must understand how it arises. In a new study, researchers from Germany, Britain, France, Spain, and Australia now use high-resolution imaging to visualize how HIV spreads between living cells at millisecond resolution. Using ultra-high resolution STED fluorescence microscopy, they provide the first direct evidence that the HIV virus builds a lipid environment for its own replication. In response, they created a method to study how this viral replication may be potentially prevented. The relevant findings were published on the journal Science Advances under the title “HIV-1 Gag specifically constraints PI (4, 5) P2 and cholesterol mobility in living cells creating a nanodomain platform for virus assembly”. The corresponding authors of the paper are Professor Christian Eggeling of the University of Jena, Germany, and Delphine Muriaux of the Institute of Infectious Diseases, Montpellier, France.
Focus on the plasma membrane of the host cell
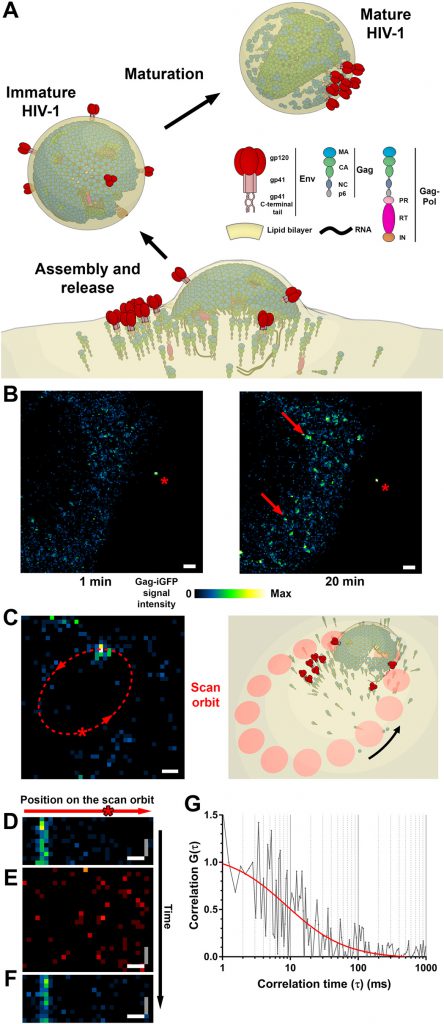
These researchers focused on the gate on the plasma membrane of the host cell through which HIV appears in the host cell after infection. They used the protein Gag, which coordinates the processes involved in HIV maturation, as a marker. “Where this protein accumulates, these critical processes cause the virus to self-release and infect other cells.” explains Eggeling.
To decipher these processes, the researchers explored the spread that occurs at the site of budding of HIV particles. They found that only certain lipids interact with the HIV virus. Although in principle these lipids are known, for the first time they were able to demonstrate this interaction directly in living infected cells.
Attack Point of Block HIV Replication
“This provides us with a potential target for the development of antiviral drugs,” Eggeling said. “Understanding which molecules are required for the HIV virus to be released from host cells and undergo replication is a critical prerequisite for studying how to prevent this viral infection. With our technology, we can now track this directly.” Eggeling and his team now want to develop antibodies that attack these molecules, thereby inhibiting the spread of the HIV virus.
Eggeling, describing his research project, said: “We need not only to study these antibodies from a medical perspective, but also to find out how to use their biophysical interactions to enhance their efficacy. To this end, we analyzed biological processes, i.e., cell-molecule interactions, with the help of physical parameters such as diffusion.”
Eggeling combines spatial super-resolution fluorescence microscopy techniques with methods that enable real-time tracking of labeled molecular movements to understand how diseases arise at the molecular level. This allowed him and his team to study individual molecules in living cells in space and time. “This allows us to uncover cellular mechanisms at the molecular level, which is much faster than previous research methods, and can operate at a small spatial scale.”
At the University of Jena, Eggeling now works closely with biologists and physicians to explore how these methods can be used to detect diseases earlier and more accurately, and may even prevent them.
Reference
1.C. Favard et al. HIV-1 Gag specifically restricts PI(4,5)P2 and cholesterol mobility in living cells creating a nanodomain platform for virus assembly. Science Advances, 2019, doi:10.1126/sciadv.aaw8651.
2.Researchers make visible how AIDS pathogens multiply in the body. https://medicalxpress.com/news/2019-10-visible-aids-pathogens-body.html