(Continued)
- PNAS: Scientists find another key protein that helps humans feel the taste
doi:10.1073/pnas.1718802115
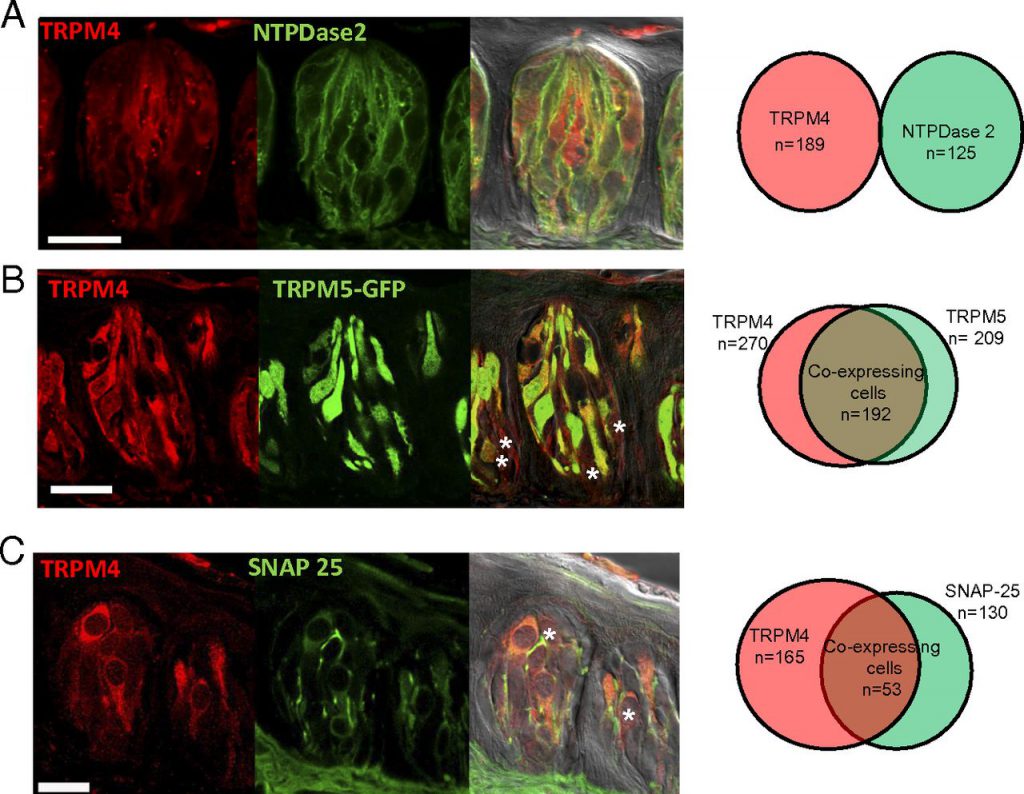
Until now, many scientists believe that a protein called TRPM5 is the key to distinguish these tastes. When TRPM5 is removed from human taste cells, they can no longer taste sweet, bitter or salty foods. The results of a recent study challenged this existing concept. According to an article recently published in PNAS, the authors discovered another important protein for the taste system called TRPM4.
In this study, the authors fed TRPM4 intact mice with sugar water, salty foods, and bitter foods. In addition, they also performed the same treatment in deficient mice. The results showed that mice lacking this type of protein were difficult to distinguish taste between sugar, salt, and bitter.
Like TRPM5, TRPM4 is also an ion channel protein. When the tongue tastes sweet, bitter, and salty foods, ion channels are opened, and electrical signals are sent to the brain, telling us what it is.
The author found that mice have the highest sensitivity to taste when both types of receptors are present in taste cells, and this sensitivity decreases significantly when any one of the proteins is removed. In the absence of both proteins, mice did not feel any taste.
- Nature: The first obtain of the three-dimensional structure of the mechanically activated ion channel Piezo1
doi:10.1038/nature25453
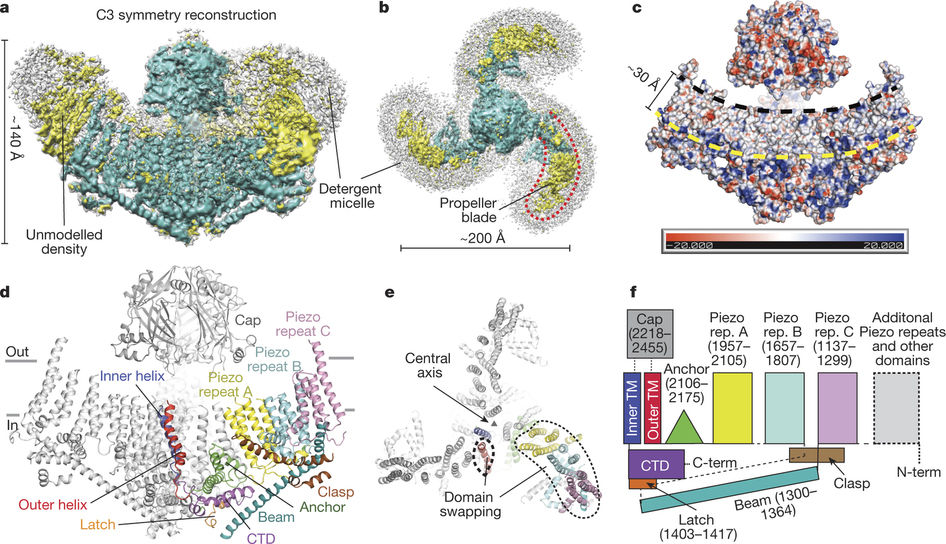
In a new study, researchers from the United States Scripps Research Institute (TSRI) solved the puzzle of the structure of Piezo1. Piezo1 is a member of a protein family that converts physical stimuli, such as touch or blood flow, into chemical signals. This finding points the way to diseases that target the treatment of mutations in Piezo1, such as hereditary oral astrocytosis and congenital lymphoedema. The relevant research results were published in Nature, and the paper is entitled “Structure of the mechanically activated ion channel Piezo1”.
Using high-resolution cryo-EM technology, this new study shows that Piezo1 consists of three curved “blade” that surround a central hole. These researchers believe that these blades move in response to mechanical forces, which opens and closes the central hole, allowing ions to send signals through this central hole to convey the touch signal. A beam-like structure serves as the skeleton of each blade. An “anchoring domain” surrounds this central hole, where the leaves meet in the middle.
- Nature: Reveals the three-dimensional structure of human epithelial calcium channel TRPV6
doi:10.1038/nature25182
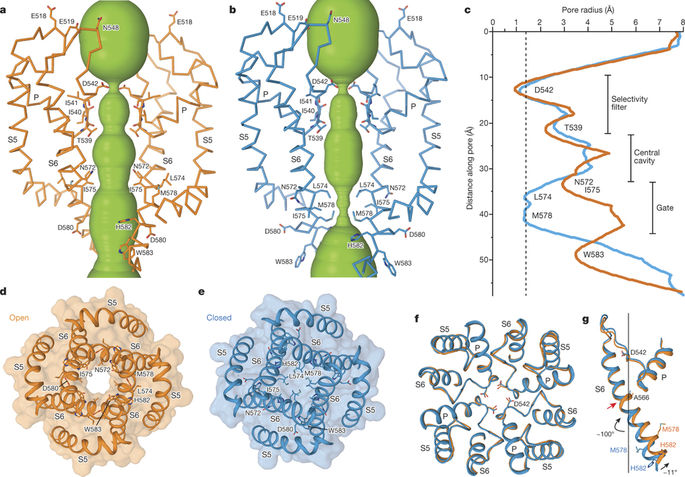
In a new study, researchers from Columbia University Medical Center for the first time obtained a detailed structural picture of a membrane pore that allows epithelial cells to absorb calcium ions. This finding may accelerate the development of drugs that correct abnormalities in calcium uptake associated with the presence of breast, endometrial, prostate, and colon cancers. The results of the relevant study were published in Nature, the paper titled “Opening of the human epithelial calcium channel TRPV6”.
The researchers used advanced cryo-electron microscopy to image TRPV6. By comparing the structure of the channel protein TRPV6 in the open and closed states, they were able to determine that the core part of this channel protein—four closely-arranged helix protein fragments—distorted slightly, allowing TRPV6 to turn on.
- Science: Obtained the structure of the TRPM8 protein which perceives cold temperature and menthol
doi:10.1126/science.aan4325
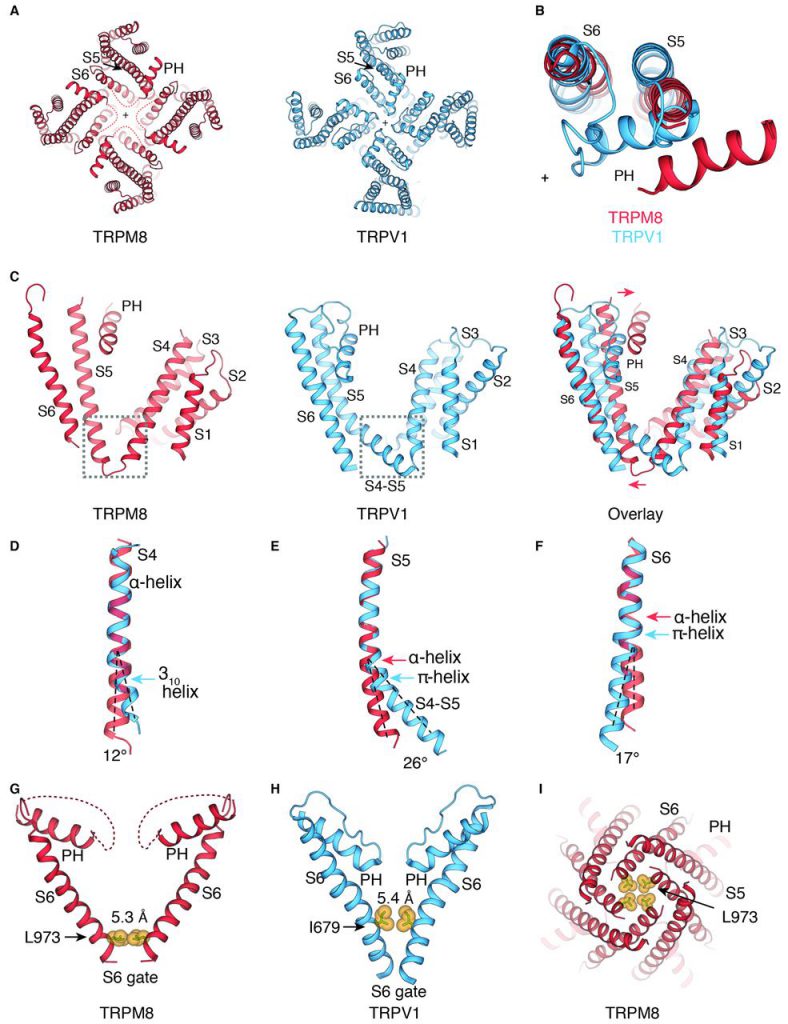
Although best known as TRPM8 is a peripheral nerve sensor that senses moderately cold temperatures (less than about 25°C) and cold-sensation molecules such as menthol. It is even among many other normal tissues, found deep in the body, but its function in these organizations is still largely unknown. A detailed understanding of the structural interactions of TRPM8 with its natural binding partner should lead to the development of better molecular probes that reveal its various functions.
For this reason, in a new study, researchers from the Scripps Research Institute and Duke University in the United States used Cryo-EM technology, a structure determination method that is increasingly favored by people. They first screened TRPM8 protein from more than a dozen different animal species (including humans, mice, and birds) in order to find a protein that might be most suitable for cryo-electron microscopy studies. They identified the protein TRPM8 from a bird species called the collared flycatcher. The relevant research results were published in Science, entitled “Structure of the cold-and-menthol-sensing ion channel TRPM8”.
- Nature: Structurally reveals TMEM16A activation mechanism and promises to develop new cystic fibrosis therapy
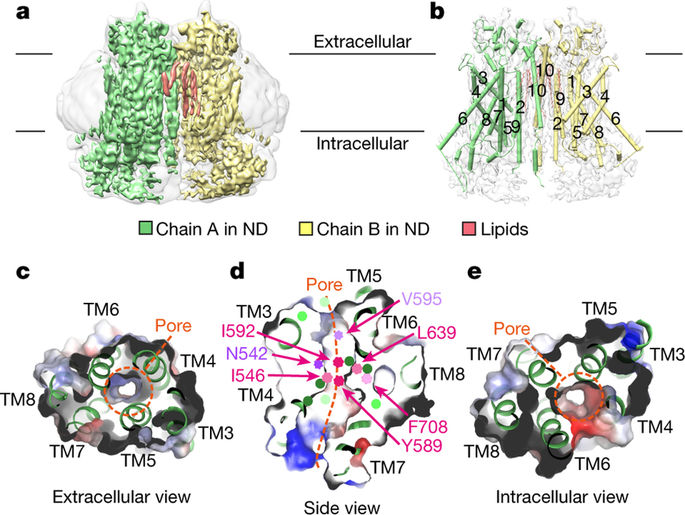
doi:10.1038/nature24652
In a new study, researchers from the University of Zurich in Switzerland used cryo-EM to resolve the detailed structure of the chloride channel TMEM16A. This protein is a promising target for the development of effective treatment of cystic fibrosis. The relevant research results were published in Nature, entitled “Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM”.
The chloride channel TMEM16A is found in various organs of the body, and plays a key role in the secretion of chloride ions in the lungs and smooth muscle contraction and pain perception. Today, these researchers used a combination of cryo-EM and electrophysiological techniques to reveal how the structure of TMEM16A differs from the enzymes that belong to the same protein family in close homologues and how it is activated by calcium ions. Although the overall structure of TMEM16A is similar to that of the enzyme belonging to the same protein family, there is a significant difference in the ion permeation pore area located in each subunit of this dimeric protein. The scramblase contains a membrane exposed polar furrow, which allows the lipid head to diffuse through the lipid bilayer. In contrast, in the same position, TMEM16A forms an hourglass-like protein-enclosed channel that is closed in the absence of calcium ions. The combination of positively charged calcium ions in the vicinity of this channel opens the channel, allowing negatively charged chloride ions to pass through the cell membrane. Cristina Paulino, the author of the paper, explained that “this activation mechanism is unique because calcium ions directly change the structure and electrostatic properties of the ion-permeable pores.”
- Science: Boosting the development of optical genetics by revealing the three-dimensional structure of channel protein 2 in rhodopsin
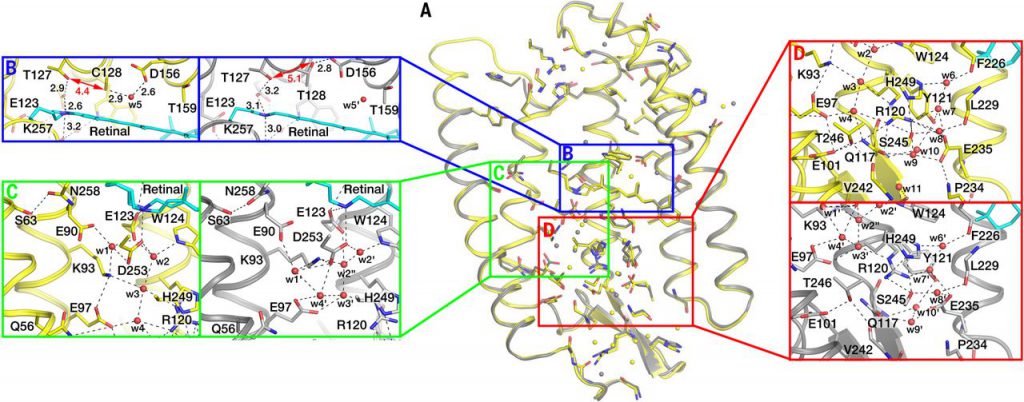
doi:10.1126/science.aan8862
Channelrhodopsin 2 (ChR2) is a membrane protein widely used in optogenetics. Optogenetics technology is a relatively new technology that involves the use of light to manipulate neurons and muscle cells in living organisms. Similar methods have been used to partially reverse hearing/vision loss and control muscle contractions.
To reveal the structure of ChR2, researchers from Germany, France, Russia, and the Czech Republic used an analysis technique X-ray diffraction, which is only used to analyze protein samples that exist in crystalline form. They culture ChR2 protein crystals in a so-called cubic lipid mesophase that allows proteins to move freely without leaving the membrane. They used X-rays with a wavelength of about 1 Angstrom to irradiate their cultured ChR2 protein crystals. The structure of the ChR2 protein was successfully resolved by analyzing the X-ray diffraction pattern in this protein crystal. The relevant research results were published in Science. The paper titled “Structural insights into ion conduction by channelrhodopsin 2”.
Reference
Dutta Banik D. et al, TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc Natl Acad Sci U S A. 2018 Jan 23;115(4):E772-E781. doi: 10.1073/pnas.1718802115
Saotome K. et al., Structure of the mechanically activated ion channel Piezo1. Nature. 2018 Feb 22;554(7693):481-486. doi: 10.1038/nature25453
McGoldrick LL. et al., Opening of the human epithelial calcium channel TRPV6. Nature. 2018 Jan 11;553(7687):233-237. doi: 10.1038/nature25182
Yin Y. et al. Structure of the cold- and menthol-sensing ion channel TRPM8. Science. 2018 Jan 12;359(6372):237-241. doi: 10.1126/science.aan4325
Paulino C. et al. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature. 2017 Dec 21;552(7685):421-425. doi: 10.1038/nature24652.
Volkov O. et al. Structural insights into ion conduction by channelrhodopsin 2. Science. 2017 Nov 24;358(6366). pii: eaan8862. doi: 10.1126/science.aan8862.
Gerwert K. Channelrhodopsin reveals its dark secrets. Science. 2017 Nov 24;358(6366):1000-1001. doi: 10.1126/science.aar2299.