Photosynthesis is a process of large-scale utilization of solar energy to synthesize carbon dioxide and water into organic compounds and release oxygen. Photosystem Ⅱ (PSⅡ) is located on the thylakoid membrane of oxygen releasing photosynthetic organisms, which is an important place for the oxidation of photosynthetic water. PSⅡ core complex has the function of photosynthesis and oxygen releasing, which is a pigment membrane protein and composed of 20 protein subunits, manganese clusters, pigment molecules, and other cofactors. Exploring the structure and function regulation mechanism of PSII is one of the frontier scientific issues in the world.
The assembly of PSⅡ in vivo and the repair process after light damage require a variety of assembly factors, which is a complex and highly ordered process through the formation of different assembly repair intermediate complexes. Psb27 protein is one of the important assembly repair factors, which plays an important role in maintaining the efficient assembly repair of PSⅡ under the stress of low temperature, high light, or rapid change of light intensity. At present, it is still a challenge to reveal the precise mechanism of Psb27 regulating PSⅡ assembly at the atomic and molecular levels.
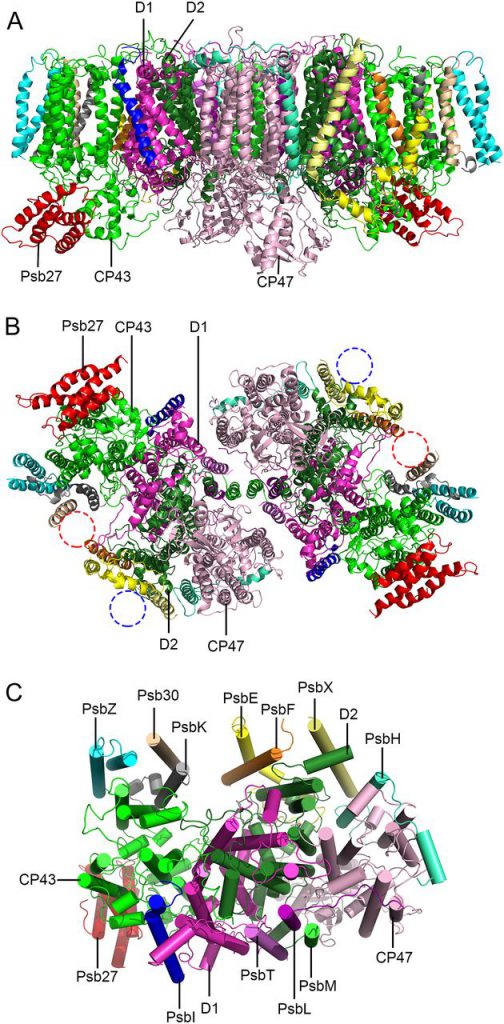
The photosynthetic membrane protein structural biology research team of the Institute of Botany, the Chinese Academy of Sciences, and Sui Senfang research team of Tsinghua University cooperated to analyze Thermosynechococcus for the first time by using single-particle freezing electron microscopy. The near-atomic resolution (3.78 Å) three-dimensional structure of the Psb27-PSⅡ intermediate complex reveals the precise binding site of Psb27 and the unique structural characteristics of the complex. It was found that the protein complex was a dimer composed of two Psb27-PSⅡ monomers according to C2 symmetry. Each Psb27-PSⅡ monomer contained 16 protein subunits, 35 chlorophyll molecules, 11 carotene molecules, 2 demethylated chlorophyll molecules, and a large number of lipid molecules. On the lumen side of PSⅡ, Psb27 protein interacts with the CP43 subunit to bind to the intermediate complex, and the binding Psb27 protein has some structural conflicts with PsbO. At the same time, it was found that some local conformations of CP43, CP47, D2 subunits, and other core subunits in the intermediate complexes changed, which prevented PsbO, PsbU, and PSⅡ complexes from binding prematurely before the assembly of manganese clusters in the catalytic center of hydrocracking.
Based on the structural information, a new model for the dissociation and binding of periproteins during PSⅡ assembly and repair was proposed. This is the first intermediate complex structure of PSⅡ, which provides an important structural basis for further revealing the assembly and repair process of PSⅡ. It is an important progress in understanding the dynamic regulation of structure and function during the assembly and repair process of PSⅡ.
Reference
Guoqiang Huang, Yanan Xiao, Xiong Pi, et al. Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus. PNAS, 2021 118 (5) e2018053118; https://doi.org/10.1073/pnas.2018053118