In a new study, researchers from the Flanders Institute for Biotechnology, the Free University of Brussels (VUB), the Eindhoven University of Technology in the Netherlands, the University of Savoy in France and the Biscay Science and Technology Park in Spain, found for the first time the molecular details of nucleation of protein crystals, which is of great medical and scientific significance.
Dr. Mike Sleutel (VIB/VUB), co-author of the paper, said, “To see this new technology in the future be applied to the process of protein self-assembly related to a series of diseases—such as liquid-liquid phase separation in cataract formation or the formation of amyloid fibrils associated with numerous neurological diseases will be exciting.”
Protein crystals are of great medical and scientific significance. For decades, they have been the key for structural biologists to resolve the three-dimensional structure of proteins, but protein crystals have also been used as biologic drug delivery agents. Protein crystal suspensions are attractive formulations for the storage and administration of active pharmaceutical compounds because of their longer shelf life, lower solvent viscosity, and slower dissolution rate. Perhaps the best-known example is insulin: Insulin injections include subcutaneous injections of insulin microcrystalline suspensions, which dissolve slowly, producing stable and sustained delivery. Despite their enormous potential, there are two factors that limit the widespread use of protein crystals.
Protein Crystal Formation Challenges
First of all, as many molecular biologists say, cultivating protein crystals is more of an art than a science. In fact, crystallization can be very difficult for many proteins. This is partly because scientists do not understand the early stages of protein crystal formation. Any crystal originates from the nucleus. The nucleus is a tiny seed that is formed by the spontaneous combination of several molecules in solution and must adopt a three-dimensional, and regular structure. How these molecules could achieve this impossible feat has been a mystery before this.
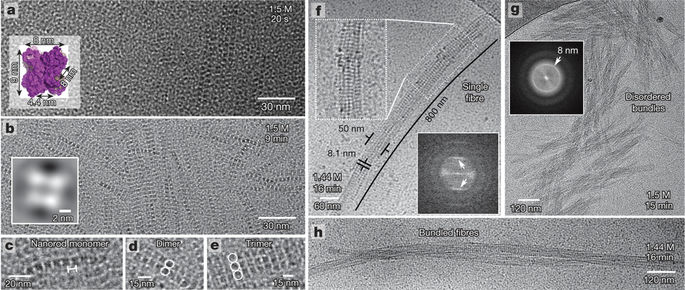
The researchers used a state-of-the-art cryo-transmission electron microscopy (Cryo-TEM) to visualize the nucleation of protein crystals at the molecular resolution to capture images of protein crystal formation.
Second, a protein can crystallize in many different crystal forms, which is called polymorphism. Different crystal forms have different characteristics, of which the most significant are the ability to diffract X-rays (which are crucial for determining the 3D structure) and the dissolution rates (which are essential for drug delivery). So far, it has been difficult to guide the crystallization process to form the crystal form that people like. Scientists believe that crystal selection takes place at the nucleation stage, but no one knows exactly how this mechanism works.
Dr. Heiner Friedrich, the co-author of the paper, explained that “because this nucleation process is happening so quickly and within such a small length, we need to capture pictures of protein samples at various stages of the process. We use a very sensitive electron microscopy technique to visualize these protein molecules and how they are combined to form nuclei and eventually form protein crystals.”
By analyzing the Cryo-TEM images of a series of captured protein samples at regular intervals, the researchers were able to stitch together a series of molecular collisions that are required for the formation of nuclei. Dr. Sleutel said, “We were shocked by the unexpected complexity of this process, and the facts confirm that this process is much more complicated than the experimental models that we and other people in this field had before these observations. For the proteins we used in this study, we have discovered a hierarchical self-assembly process that involves the subsequent three stages of self-assembly, during which the size of the protein crystals continues to increase. These observations provide a new method for studying the self-assembly of macromolecules into larger structures.
However, these researchers conducted further research and compared the nucleation pathways of various crystal types. They showed that the choice of crystal form depends on the structure of the smallest possible fragment that was formed early. Once this structure is formed, this protein crystal nucleation process is established. Thesis co-author Dr. Alexander Van Driessche explained, “By analyzing and understanding the differences in the structure of different nuclei, we have developed strategies to guide the process of selecting crystals. We have moderately adjusted the different modes of interaction between protein molecules. This is achieved by manipulating this nucleation process in the way we choose.” They believe that these new insights and methods will significantly accelerate the development of protein crystals in three-dimensional structure determination and medical applications.
Reference
Alexander E. S. Van Driessche, Nani Van Gerven, Paul H. H. Bomans et al. Molecular nucleation mechanisms and control strategies for crystal polymorph selection. Nature, 5 April 2018, 556(7699):89–94, doi:10.1038/nature25971