Single-molecule optical imaging can analyze the morphology and function of a single molecule, so it can not only provide statistical average information of multiple molecules, but also provide single-molecule individual difference information that traditional biosensors cannot provide, allowing us to observe and understand the nature and function of the molecule in the more detail.
The research team of Professor NJ Tao and Professor Shaopeng Wang of the Bioelectronics and Biosensing Research Center of Arizona State University’s Biodesign Institute published a new type of optical microscope imaging system for labeling and detecting individual protein molecules.
Surface Plasmon Resonance (SPR) is an optical detection technique that has been widely used in the measurement of label-free molecular interaction dynamics. The SPR microscope developed in recent years has improved the spatial resolution of SPR detection and realized the imaging analysis of single exosomes and viruses. However, the realization of SPR microscopic imaging with single-molecule precision still has great challenges.
Although Professor Tao and Professor Wang predicted 20 years ago that if the incident light is strong enough, SPR can detect a single protein molecule, but because the traditional SPR microscope detects reflected light, the background light is relatively strong, and using the current high-speed camera has been overexposed even under incident light irradiation conditions of the intensity level of one hundred watts per square centimeter. On the other hand, because the general size of the protein molecule is on the order of 10 nanometers, its scattering cross section is very small, so the detection signal is easily annihilated by environmental noise.
The research team overcomes the above technical difficulties by using a second objective lens on a traditional SPR microscope to collect and detect plasmon scattered light. This technique is named Plasmonic Scattering Microscopy (PSM).
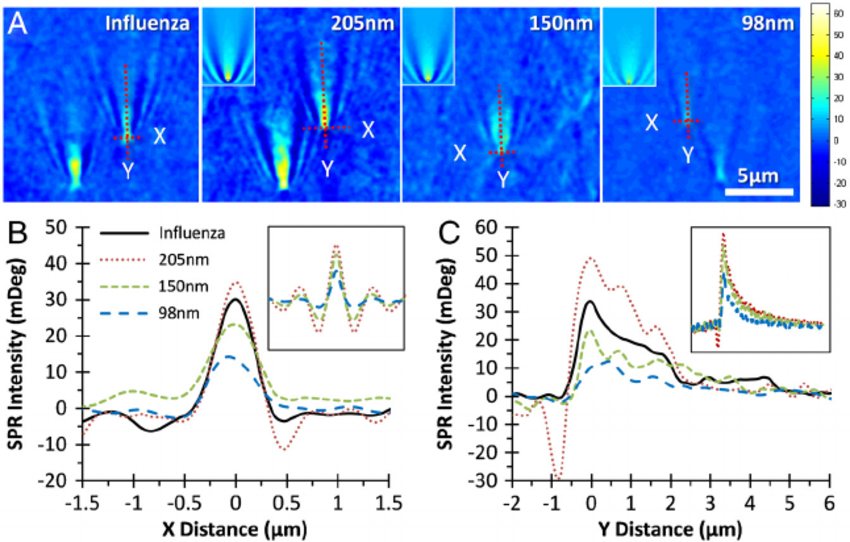
The PSM microscope avoids the collection of reflected light and thus can use incident light with an intensity of kilowatts per square centimeter for detection, which is thousands of times the intensity of incident light used by traditional SPR microscopes. This provides an astigmatic light signal of sufficient intensity for high-speed imaging, allowing the average noise reduction and differential imaging processing of multiple image data under the premise of ensuring the time resolution, which can reduce the impact of environmental noise to an acceptable level.
In addition, after subtracting the background scattering caused by rough particles on the gold film surface, it can be seen that there is no parabolic tail caused by the non-local interference of the surface plasmon plane in the traditional SPR microscopic image in the PSM image. Improve the contrast of the image and greatly reduce the difficulty of data processing.
When using a PSM microscope for imaging, the signal intensity change trend can be divided into two. One is that when an object with a scale of more than 100 nanometers is detected, the signal intensity changes with the sixth power of the object’s diameter. This is because the scattering intensity of such objects is much greater than the background scattering of rough particles on the surface of the gold film, and the signal is dominated by the scattering. The second is that when detecting objects with a scale of smaller than 100 nanometers, the background scattering of rough particles on the gold film surface is much larger than that of the analyte, which presents an obvious interference effect, and the signal intensity changes with the cube of the diameter.
Because the size of protein molecules is generally less than 30 nanometers, when the PSM microscope is used for protein detection, its signal intensity changes as a typical interference signal. Surface rough particle scattering, which is regarded as a noise source that is difficult to eliminate in most imaging applications, has been successfully used in PSM microscopes to amplify the interference reference light of the detected object signal.
Utilizing the single-molecule optical imaging capabilities of the PSM microscope, not only can the molecular counting method be used to obtain molecular dynamics curves similar to those obtained by traditional SPR detection, but also single protein molecules and antibody modifications can be detected. The heterogeneity of the surface interaction process of the gold film provides more detailed information for molecular dynamics analysis. In addition, because the PSM microscope is built on the SPR microscope, the PSM microscope still retains the ability to perform surface electrochemical monitoring, ion detection, biochemical reaction monitoring, and other common application research capabilities of SPR.
This paper verifies the ability of the PSM microscope constructed based on the SPR microscope to perform single-molecule optical imaging and verifies the application feasibility of the PSM microscope in the measurement of non-labeled protein molecular interaction dynamics. As a new member of the SPR sensor family, the PSM microscope is related to the study of the specific process of the interaction between the scattering of surface rough particles and the scattering of the analyte, and the principle study of the details of the non-locality of the surface plasmon plane, as well as single-molecule optics imaging capabilities and the deep integration of various application fields of SPR. PSM will become a new topic in the field of SPR imaging detection and has broad research and development prospects.