In an article published on “eLife”, researchers at the University of Delaware and Indiana University found that the details of the unknown hepatitis B virus capsid, illuminating potentially new therapies for more than 250 million hepatitis B patients worldwide.
The capsids play the role of hepatitis B virus in injecting genetic material into host cells. They are a key target for the development of therapeutic drugs for hepatitis B.
“For hepatitis B, the capsid structure of the virus has been reported for a long time. Our starting point is to study the movement of the capsid and its impact on the surrounding environment,” said Jodi A. Hadden, researcher of the Department of Chemistry and Biochemistry.
Hadden and her research team used supercomputer resources to perform “atomic molecular dynamics simulations.”
Molecular dynamics simulation allows researchers to observe how molecules move to understand their function in nature. Computer simulation is the only method that can reveal the motion of molecular systems at the atomic level. This method is also called “computing microscopy.”
In simulating hepatitis B virus, the researchers found that the capsids were not as stiff as previously thought, they were actually quite flexible. It can be distorted into asymmetrical shapes and can even be squeezed directly through an opening into the nucleus of a virus-infected cell.
“We believe that the distorting ability of the capsid is necessary to properly package the genetic information into the nucleus and eventually produce new copies of the virus during infection,” said Hadden.
Early experimental microscope observations showed that these tiny capsids consist of 240 proteins, but previous studies could not produce high-resolution images of more complex structures, said Juan R. Perilla, assistant professor of chemistry and biochemistry.
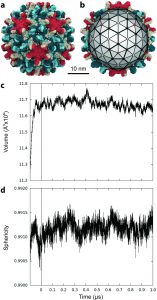
“Obviously, the HBV flexibility test is a limitation of the microscope,” he said. “In contrast, analog technology has been able to reveal a more complete picture of the capsid, in addition, can provide information on how they move, twist and interact with the environment.” A single simulation includes 6 million atoms of information.
“We have analyzed all the details to the atom level,” Hadden said. “To study drug interactions, firstly, you must fully understand it.”
The researchers found that the small triangular openings (or pores) on the surface of the capsid may be the position where its protein “tail” pierces, which is an important sign of infection.
“We have always known that the tail of the capsid must be exposed on the surface for a period of time before the capsid can enter the nucleus,” Hadden said. “We call this behavior of the virus as ‘call a taxi.’”
These findings are likely to translate into drug treatment target. For example, to prevent deformation of the capsid, make them hard, or block the surface of the triangle hole, it may block the infection process.
Although the HBV vaccine can prevent it, it cannot be cured once it is infected. Given that hepatitis B virus can cause serious liver disease and even life-threatening, researchers are trying to block them in the body and eventually achieve a cure.
Reference
Jodi A Hadden et al. All-atom molecular dynamics of the HBV capsid reveals insights into biological function and cryo-EM resolution limits. eLife 2018;7:e32478 DOI: 10.7554/eLife.32478